ZccE is a Novel P-type ATPase That Protects Streptococcus mutans Against Zinc Intoxication
- PMID: 35939512
- PMCID: PMC9387928
- DOI: 10.1371/journal.ppat.1010477
ZccE is a Novel P-type ATPase That Protects Streptococcus mutans Against Zinc Intoxication
Abstract
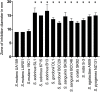
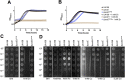
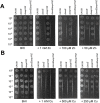
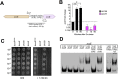
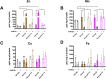
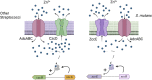
Zinc is a trace metal that is essential to all forms of life, but that becomes toxic at high concentrations. Because it has both antimicrobial and anti-inflammatory properties and low toxicity to mammalian cells, zinc has been used as a therapeutic agent for centuries to treat a variety of infectious and non-infectious conditions. While the usefulness of zinc-based therapies in caries prevention is controversial, zinc is incorporated into toothpaste and mouthwash formulations to prevent gingivitis and halitosis. Despite this widespread use of zinc in oral healthcare, the mechanisms that allow Streptococcus mutans, a keystone pathogen in dental caries and prevalent etiological agent of infective endocarditis, to overcome zinc toxicity are largely unknown. Here, we discovered that S. mutans is inherently more tolerant to high zinc stress than all other species of streptococci tested, including commensal streptococci associated with oral health. Using a transcriptome approach, we uncovered several potential strategies utilized by S. mutans to overcome zinc toxicity. Among them, we identified a previously uncharacterized P-type ATPase transporter and cognate transcriptional regulator, which we named ZccE and ZccR respectively, as responsible for the remarkable high zinc tolerance of S. mutans. In addition to zinc, we found that ZccE, which was found to be unique to S. mutans strains, mediates tolerance to at least three additional metal ions, namely cadmium, cobalt, and copper. Loss of the ability to maintain zinc homeostasis when exposed to high zinc stress severely disturbed zinc:manganese ratios, leading to heightened peroxide sensitivity that was alleviated by manganese supplementation. Finally, we showed that the ability of the ΔzccE strain to stably colonize the rat tooth surface after topical zinc treatment was significantly impaired, providing proof of concept that ZccE and ZccR are suitable _targets for the development of antimicrobial therapies specifically tailored to kill S. mutans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



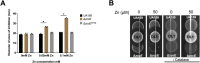

Similar articles
-
ZccE, a P-type ATPase contributing to biofilm formation and competitiveness in Streptococcus mutans.Mol Oral Microbiol. 2023 Jun;38(3):198-211. doi: 10.1111/omi.12405. Epub 2023 Jan 28. Mol Oral Microbiol. 2023. PMID: 36622758
-
Zinc import mediated by AdcABC is critical for colonization of the dental biofilm by Streptococcus mutans in an animal model.Mol Oral Microbiol. 2021 Jun;36(3):214-224. doi: 10.1111/omi.12337. Epub 2021 May 10. Mol Oral Microbiol. 2021. PMID: 33819383 Free PMC article.
-
Effects of Antimicrobial Peptide GH12 on the Cariogenic Properties and Composition of a Cariogenic Multispecies Biofilm.Appl Environ Microbiol. 2018 Nov 30;84(24):e01423-18. doi: 10.1128/AEM.01423-18. Print 2018 Dec 15. Appl Environ Microbiol. 2018. PMID: 30341079 Free PMC article.
-
[Secondary metabolites from Streptococcus mutans and their ecological roles in dental biofilm].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1547-1554. doi: 10.13345/j.cjb.170046. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956400 Review. Chinese.
-
Inhibition of Streptococcus mutans biofilm formation by strategies _targeting the metabolism of exopolysaccharides.Crit Rev Microbiol. 2021 Sep;47(5):667-677. doi: 10.1080/1040841X.2021.1915959. Epub 2021 May 3. Crit Rev Microbiol. 2021. PMID: 33938347 Review.
Cited by
-
Catalase produced by Candida albicans protects Streptococcus mutans from H2O2 stress-one more piece in the cross-kingdom synergism puzzle.mSphere. 2023 Oct 24;8(5):e0029523. doi: 10.1128/msphere.00295-23. Epub 2023 Aug 21. mSphere. 2023. PMID: 37607054 Free PMC article.
-
Oxidative stress response: a critical factor affecting the ecological competitiveness of Streptococcus mutans.J Oral Microbiol. 2023 Dec 12;16(1):2292539. doi: 10.1080/20002297.2023.2292539. eCollection 2024. J Oral Microbiol. 2023. PMID: 38405599 Free PMC article. Review.
-
A mouthwash formulated with o-cymen-5-ol and zinc chloride specifically _targets potential pathogens without impairing the native oral microbiome in healthy individuals.J Oral Microbiol. 2023 Mar 3;15(1):2185962. doi: 10.1080/20002297.2023.2185962. eCollection 2023. J Oral Microbiol. 2023. PMID: 36891194 Free PMC article.
-
Metal Homeostasis in Pathogenic Streptococci.Microorganisms. 2022 Jul 25;10(8):1501. doi: 10.3390/microorganisms10081501. Microorganisms. 2022. PMID: 35893559 Free PMC article. Review.
-
Advances in stress-tolerance elements for microbial cell factories.Synth Syst Biotechnol. 2024 Jun 28;9(4):793-808. doi: 10.1016/j.synbio.2024.06.008. eCollection 2024 Dec. Synth Syst Biotechnol. 2024. PMID: 39072145 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

