PEDOT: PSS promotes neurogenic commitment of neural crest-derived stem cells
- PMID: 36060701
- PMCID: PMC9428488
- DOI: 10.3389/fphys.2022.930804
PEDOT: PSS promotes neurogenic commitment of neural crest-derived stem cells
Abstract
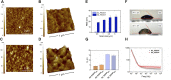
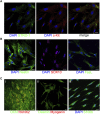
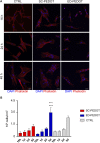
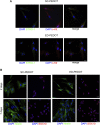
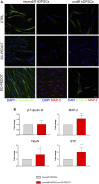
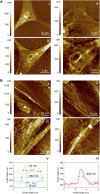
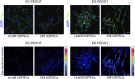
Poly (3,4-ethylendioxythiophene) polystyrene sulphonate (PEDOT:PSS) is the workhorse of organic bioelectronics and is steadily gaining interest also in tissue engineering due to the opportunity to endow traditional biomaterials for scaffolds with conductive properties. Biomaterials capable of promoting neural stem cell differentiation by application of suitable electrical stimulation protocols are highly desirable in neural tissue engineering. In this study, we evaluated the adhesion, proliferation, maintenance of neural crest stemness markers and neurogenic commitment of neural crest-derived human dental pulp stem cells (hDPSCs) cultured on PEDOT:PSS nanostructured thin films deposited either by spin coating (SC-PEDOT) or by electropolymerization (ED-PEDOT). In addition, we evaluated the immunomodulatory properties of hDPSCs on PEDOT:PSS by investigating the expression and maintenance of the Fas ligand (FasL). We found that both SC-PEDOT and ED-PEDOT thin films supported hDPSCs adhesion and proliferation; however, the number of cells on the ED-PEDOT after 1 week of culture was significantly higher than that on SC-PEDOT. To be noted, both PEDOT:PSS films did not affect the stemness phenotype of hDPSCs, as indicated by the maintenance of the neural crest markers Nestin and SOX10. Interestingly, neurogenic induction was clearly promoted on ED-PEDOT, as indicated by the strong expression of MAP-2 and -Tubulin-III as well as evident cytoskeletal reorganisation and appreciable morphology shift towards a neuronal-like shape. In addition, strong FasL expression was detected on both undifferentiated or undergoing neurogenic commitment hDPSCs, suggesting that ED-PEDOT supports the expression and maintenance of FasL under both expansion and differentiation conditions.
Keywords: cell differentiation; conductive polymers; dental pulp stem cells; immunomodulatory properties; nanostructured thin films; stemness.
Copyright © 2022 Pisciotta, Lunghi, Bertani, Di Tinco, Bertoni, Orlandi, Biscarini, Bianchi and Carnevale.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Use of a 3D Floating Sphere Culture System to Maintain the Neural Crest-Related Properties of Human Dental Pulp Stem Cells.Front Physiol. 2018 May 28;9:547. doi: 10.3389/fphys.2018.00547. eCollection 2018. Front Physiol. 2018. PMID: 29892229 Free PMC article.
-
BDNF and NT3 Reprogram Human Ectomesenchymal Dental Pulp Stem Cells to Neurogenic and Gliogenic Neural Crest Progenitors Cultured in Serum-Free Medium.Cell Physiol Biochem. 2019;52(6):1361-1380. doi: 10.33594/000000096. Cell Physiol Biochem. 2019. PMID: 31075188
-
Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering.Biochim Biophys Acta. 2015 Jun;1850(6):1158-68. doi: 10.1016/j.bbagen.2015.01.020. Epub 2015 Feb 7. Biochim Biophys Acta. 2015. PMID: 25662071
-
Directly Induced Neural Differentiation of Human Adipose-Derived Stem Cells Using Three-Dimensional Culture System of Conductive Microwell with Electrical Stimulation.Tissue Eng Part A. 2018 Apr;24(7-8):537-545. doi: 10.1089/ten.TEA.2017.0150. Epub 2017 Aug 31. Tissue Eng Part A. 2018. PMID: 28741412
-
3D organic bioelectronics for electrical monitoring of human adult stem cells.Mater Horiz. 2023 Aug 29;10(9):3589-3600. doi: 10.1039/d3mh00785e. Mater Horiz. 2023. PMID: 37318042 Free PMC article.
Cited by
-
Electroactive Polymers for On-Demand Drug Release.Adv Healthc Mater. 2024 Jan;13(3):e2301759. doi: 10.1002/adhm.202301759. Epub 2023 Nov 12. Adv Healthc Mater. 2024. PMID: 37861058 Free PMC article. Review.
-
Biomarkers of mature neuronal differentiation and related diseases.Future Sci OA. 2024 Dec 31;10(1):2410146. doi: 10.1080/20565623.2024.2410146. Epub 2024 Oct 21. Future Sci OA. 2024. PMID: 39429212 Free PMC article. Review.
-
Editorial: Advanced neural stem cell therapies for spinal cord injury.Front Pharmacol. 2024 Aug 6;15:1469535. doi: 10.3389/fphar.2024.1469535. eCollection 2024. Front Pharmacol. 2024. PMID: 39166116 Free PMC article. No abstract available.
-
Human dental pulp stem cells (hDPSCs) promote the lipofibroblast transition in the early stage of a fibro-inflammatory process.Front Cell Dev Biol. 2023 May 3;11:1196023. doi: 10.3389/fcell.2023.1196023. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37206922 Free PMC article.
-
Interface-Mediated Neurogenic Signaling: The Impact of Surface Geometry and Chemistry on Neural Cell Behavior for Regenerative and Brain-Machine Interfacing Applications.Adv Mater. 2024 Aug;36(33):e2401750. doi: 10.1002/adma.202401750. Epub 2024 Jul 3. Adv Mater. 2024. PMID: 38961531 Review.
References
-
- Arbring Sjöström T., Berggren M., Gabrielsson E. O., Janson P., Poxson D. J., Seitanidou M., et al. (2018). A decade of iontronic delivery devices. Adv. Mat. Technol. 3 (5), 1700360. 10.1002/admt.201700360 - DOI
-
- Asplund M., Nyberg T., Inganäs O. (2010). Electroactive polymers for neural interfaces. Polym. Chem. 1 (9), 1374–1391. 10.1039/C0PY00077A - DOI
-
- Bhatt V. D., Teymouri S., Melzer K., Abdellah A., Guttenberg Z., Lugli P. (2016). Biocompatibility tests on spray coated carbon nanotube and PEDOT:PSS thin films. IEEE Trans. Nanotechnol. 15 (3), 373–379. 10.1109/TNANO.2016.2535780 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

