Clinical Assessment of Fetal Well-Being and Fetal Safety Indicators
- PMID: 36106777
- PMCID: PMC9544851
- DOI: 10.1002/jcph.2126
Clinical Assessment of Fetal Well-Being and Fetal Safety Indicators
Abstract
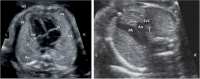
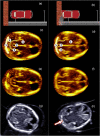
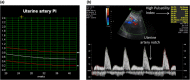
Delivering safe clinical trials of novel therapeutics is central to enable pregnant women and their babies to access medicines for better outcomes. This review describes clinical monitoring of fetal well-being and safety. Current pregnancy surveillance includes regular antenatal checks of blood pressure and urine for signs of gestational hypertension. Fetal and placental development is assessed routinely using the first-trimester "dating" and mid-trimester "anomaly" ultrasound scans, but the detection of fetal anomalies can continue throughout pregnancy using _targeted sonography or magnetic resonance imaging (MRI). Serial sonography can be used to assess fetal size, well-being, and placental function. Carefully defined reproducible imaging parameters, such as the head circumference (HC), abdominal circumference (AC), and femur length (FL), are combined to calculate an estimate of the fetal weight. Doppler analysis of maternal uterine blood flow predicts placental insufficiency, which is associated with poor fetal growth. Fetal doppler analysis can indicate circulatory decompensation and fetal hypoxia, requiring delivery to be expedited. Novel ways to assess fetal well-being and placental function using MRI, computerized cardiotocography (CTG), serum circulating fetoplacental proteins, and mRNA may improve the assessment of the safety and efficacy of maternal and fetal interventions. Progress has been made in how to define and grade clinical trial safety in pregnant women, the fetus, and neonate. A new system for improved safety monitoring for clinical trials in pregnancy, Maternal and Fetal Adverse Event Terminology (MFAET), describes 12 maternal and 18 fetal adverse event (AE) definitions and severity grading criteria developed through an international modified Delphi consensus process. This fills a vital gap in maternal and fetal translational medicine research.
Keywords: adverse event; clinical trial; fetal therapy; fetus; pregnancy; safety.
© 2022 The Authors. The Journal of Clinical Pharmacology published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
A.L.D. receives consulting fees from Esperare Foundation, Geneva, Switzerland, a private not‐for‐profit organization, as chair of the Data Safety Monitoring Board in a clinical trial of an investigational fetal drug therapy. She is an unpaid co‐chair of the Maternal Health Project Group of the Association of British Pharmaceutical Industry (ABPI). She is a commissioner (unpaid) on the University of Birmingham's Policy Commission on Safe, Effective and Accessible Medicines for Use in Pregnancy. R.N.S. has no financial interests to disclose.
Figures

Similar articles
-
Fetal growth restriction and intra-uterine growth restriction: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians.Eur J Obstet Gynecol Reprod Biol. 2015 Oct;193:10-8. doi: 10.1016/j.ejogrb.2015.06.021. Epub 2015 Jul 2. Eur J Obstet Gynecol Reprod Biol. 2015. PMID: 26207980
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Prenatal screening for fetal aneuploidy in singleton pregnancies.J Obstet Gynaecol Can. 2011 Jul;33(7):736-750. doi: 10.1016/S1701-2163(16)34961-1. J Obstet Gynaecol Can. 2011. PMID: 21749752
-
Obstetrical complications associated with abnormal maternal serum markers analytes.J Obstet Gynaecol Can. 2008 Oct;30(10):918-932. doi: 10.1016/S1701-2163(16)32973-5. J Obstet Gynaecol Can. 2008. PMID: 19038077 Review. English, French.
-
Improving Development of Drug Treatments for Pregnant Women and the Fetus.Ther Innov Regul Sci. 2022 Nov;56(6):976-990. doi: 10.1007/s43441-022-00433-w. Epub 2022 Jul 25. Ther Innov Regul Sci. 2022. PMID: 35881237 Free PMC article. Review.
Cited by
-
The neonatal adverse event severity scale: current status, a stakeholders' assessment, and future perspectives.Front Pediatr. 2024 Jan 8;11:1340607. doi: 10.3389/fped.2023.1340607. eCollection 2023. Front Pediatr. 2024. PMID: 38259600 Free PMC article.
-
Navigating the Complexity: A Comprehensive Review of Managing Pregnancy in Complete Heart Block Cases.Cureus. 2023 Dec 23;15(12):e50977. doi: 10.7759/cureus.50977. eCollection 2023 Dec. Cureus. 2023. PMID: 38259400 Free PMC article. Review.
-
Trends in research related to fetal therapy from 2012 to 2022: a bibliometric analysis.Front Pediatr. 2024 Jan 4;11:1288660. doi: 10.3389/fped.2023.1288660. eCollection 2023. Front Pediatr. 2024. PMID: 38293659 Free PMC article.
-
A Theoretical Exploration of Artificial Intelligence's Impact on Feto-Maternal Health from Conception to Delivery.Int J Womens Health. 2024 May 22;16:903-915. doi: 10.2147/IJWH.S454127. eCollection 2024. Int J Womens Health. 2024. PMID: 38800118 Free PMC article. Review.
-
Antepartum Fetal Surveillance and Optimal Timing of Delivery in Diabetic Women: A Narrative Review.J Clin Med. 2024 Jan 5;13(2):313. doi: 10.3390/jcm13020313. J Clin Med. 2024. PMID: 38256447 Free PMC article. Review.
References
-
- US Department of Health and Human Services . Protection of Human Subjects. Title 45 Code of Federal Federal Regulations Part 46, Revised January 15, 2009.
-
- University of Birmingham . Safe and Effective Medicines for Use in Pregnancy: A Call to Action. 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

