Passage number affects differentiation of sensory neurons from human induced pluripotent stem cells
- PMID: 36151116
- PMCID: PMC9508090
- DOI: 10.1038/s41598-022-19018-6
Passage number affects differentiation of sensory neurons from human induced pluripotent stem cells
Abstract
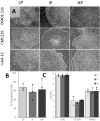
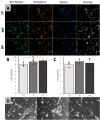
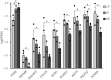
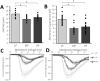
Induced pluripotent stem cells (iPSCs) are a valuable resource for neurological disease-modeling and drug discovery due to their ability to differentiate into neurons reflecting the genetics of the patient from which they are derived. iPSC-derived cultures, however, are highly variable due to heterogeneity in culture conditions. We investigated the effect of passage number on iPSC differentiation to optimize the generation of sensory neurons (iPSC-dSNs). Three iPSC lines reprogrammed from the peripheral blood of three donors were differentiated into iPSC-dSNs at passage numbers within each of the following ranges: low (5-10), intermediate (20-26), and high (30-38). Morphology and pluripotency of the parent iPSCs were assessed prior to differentiation. iPSC-dSNs were evaluated based on electrophysiological properties and expression of key neuronal markers. All iPSC lines displayed similar morphology and were similarly pluripotent across passage numbers. However, the expression levels of neuronal markers and sodium channel function analyses indicated that iPSC-dSNs differentiated from low passage numbers better recapitulated the sensory neuron phenotype than those differentiated from intermediate or high passage numbers. Our results demonstrate that lower passage numbers may be better suited for differentiation into peripheral sensory neurons.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Do Induced Pluripotent Stem Cell Characteristics Correlate with Efficient In Vitro Smooth Muscle Cell Differentiation? A Comparison of Three Patient-Derived Induced Pluripotent Stem Cell Lines.Stem Cells Dev. 2018 Oct 15;27(20):1438-1448. doi: 10.1089/scd.2018.0031. Epub 2018 Aug 28. Stem Cells Dev. 2018. PMID: 30153084
-
Extended passaging increases the efficiency of neural differentiation from induced pluripotent stem cells.BMC Neurosci. 2011 Aug 10;12:82. doi: 10.1186/1471-2202-12-82. BMC Neurosci. 2011. PMID: 21831300 Free PMC article.
-
How to differentiate induced pluripotent stem cells into sensory neurons for disease modelling: a functional assessment.Stem Cell Res Ther. 2024 Apr 5;15(1):99. doi: 10.1186/s13287-024-03696-2. Stem Cell Res Ther. 2024. PMID: 38581069 Free PMC article.
-
Recent advances for using human induced-pluripotent stem cells as pain-in-a-dish models of neuropathic pain.Exp Neurol. 2022 Dec;358:114223. doi: 10.1016/j.expneurol.2022.114223. Epub 2022 Sep 12. Exp Neurol. 2022. PMID: 36100046 Review.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
Cited by
-
Assessment of Genetic Stability in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Using Droplet Digital PCR.Int J Mol Sci. 2024 Jan 16;25(2):1101. doi: 10.3390/ijms25021101. Int J Mol Sci. 2024. PMID: 38256178 Free PMC article.
-
Progress and challenges in directing the differentiation of human iPSCs into spinal motor neurons.Front Cell Dev Biol. 2023 Jan 5;10:1089970. doi: 10.3389/fcell.2022.1089970. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684437 Free PMC article. Review.
-
A Study on iPSC-Associated Factors in the Generation of Hepatocytes.Tissue Eng Regen Med. 2024 Dec;21(8):1245-1254. doi: 10.1007/s13770-024-00674-w. Epub 2024 Nov 4. Tissue Eng Regen Med. 2024. PMID: 39495460
-
Optimization of a human induced pluripotent stem cell-derived sensory neuron model for the in vitro evaluation of taxane-induced neurotoxicity.Sci Rep. 2024 Aug 17;14(1):19075. doi: 10.1038/s41598-024-69280-z. Sci Rep. 2024. PMID: 39154055 Free PMC article.
-
Transition from Animal-Based to Human Induced Pluripotent Stem Cells (iPSCs)-Based Models of Neurodevelopmental Disorders: Opportunities and Challenges.Cells. 2023 Feb 7;12(4):538. doi: 10.3390/cells12040538. Cells. 2023. PMID: 36831205 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

