Tetratricopeptide repeat domain 36 deficiency mitigates renal tubular injury by inhibiting TGF-β1-induced epithelial-mesenchymal transition in a mouse model of chronic kidney disease
- PMID: 36157495
- PMCID: PMC9485203
- DOI: 10.1016/j.gendis.2021.04.005
Tetratricopeptide repeat domain 36 deficiency mitigates renal tubular injury by inhibiting TGF-β1-induced epithelial-mesenchymal transition in a mouse model of chronic kidney disease
Abstract
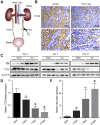
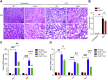
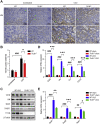
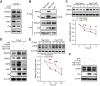
The damage of proximal tubular epithelial cells (PTECs) is considered a central event in the pathogenesis of chronic kidney disease (CKD) and deregulated repair processes of PTECs result in epithelial-mesenchymal transition (EMT), which in turn aggravates tubular injury and kidney fibrosis. In this study, we firstly revealed that the reduction of TTC36 is associated with unilateral ureteral obstruction (UUO)-induced CKD; besides, ablation of TTC36 attenuated tubular injury and subsequent EMT in UUO-treated mice kidneys. Consistently, TTC36 overexpression promoted EMT in TGF-β1-induced HK2 cells. Moreover, TTC36 elevated the protein expression of CEBPB, which was involved in the regulation of TGF-β/SMAD3 signaling, and augmented SMAD3 signaling and downstream genetic response were reduced by CEBPB silencing. Collectively, our results uncovered that TTC36 deficiency plays a protective role in tubular injury and renal fibrosis triggered by UUO; further, TTC36 overexpression exacerbated TGF-β/SMAD3 signaling via elevating the stability of SMAD3 and CEBPB, suggesting that TTC36 inhibition may be a potential strategy in the therapy of obstructive nephropathy.
Keywords: CCAAT enhancer binding protein beta; Chronic kidney disease; Epithelial−mesenchymal transition; Renal fibrosis; SMAD family bember 3; Tetratricopeptide repeat domain 36.
© 2021 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures

Similar articles
-
Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis.Stem Cell Res Ther. 2020 Sep 29;11(1):422. doi: 10.1186/s13287-020-01932-z. Stem Cell Res Ther. 2020. PMID: 32993806 Free PMC article.
-
Adenovirus-mediated P311 ameliorates renal fibrosis through inhibition of epithelial-mesenchymal transition via TGF-β1-Smad-ILK pathway in unilateral ureteral obstruction rats.Int J Mol Med. 2018 May;41(5):3015-3023. doi: 10.3892/ijmm.2018.3485. Epub 2018 Feb 12. Int J Mol Med. 2018. PMID: 29436600
-
_targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction.J Clin Invest. 2003 Nov;112(10):1486-94. doi: 10.1172/JCI19270. J Clin Invest. 2003. PMID: 14617750 Free PMC article.
-
Tetratricopeptide repeat domain 36 protects renal tubular cells from cisplatin-induced apoptosis potentially via maintaining mitochondrial homeostasis.Tissue Cell. 2022 Jun;76:101749. doi: 10.1016/j.tice.2022.101749. Epub 2022 Feb 1. Tissue Cell. 2022. PMID: 35176677
-
The glucagon-like peptide-1 (GLP-1) analog liraglutide attenuates renal fibrosis.Pharmacol Res. 2018 May;131:102-111. doi: 10.1016/j.phrs.2018.03.004. Epub 2018 Mar 9. Pharmacol Res. 2018. PMID: 29530599
Cited by
-
Co-expression of fibrotic genes in inflammatory bowel disease; A localized event?Front Immunol. 2022 Dec 23;13:1058237. doi: 10.3389/fimmu.2022.1058237. eCollection 2022. Front Immunol. 2022. PMID: 36632136 Free PMC article.
-
Astragaloside IV Blunts Epithelial-Mesenchymal Transition and G2/M Arrest to Alleviate Renal Fibrosis via Regulating ALDH2-Mediated Autophagy.Cells. 2023 Jul 4;12(13):1777. doi: 10.3390/cells12131777. Cells. 2023. PMID: 37443810 Free PMC article.
References
-
- Eddy A.A., Neilson E.G. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17(11):2964–2966. - PubMed
-
- Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. - PubMed
-
- Fernandez I.E., Eickelberg O. The impact of TGF-β on lung fibrosis: from _targeting to biomarkers. Proc Am Thorac Soc. 2012;9(3):111–116. - PubMed
LinkOut - more resources
Full Text Sources

