Effect of intravenous almitrine on intubation or mortality in patients with COVID-19 acute hypoxemic respiratory failure: A multicentre, randomised, double-blind, placebo-controlled trial
- PMID: 36157895
- PMCID: PMC9489996
- DOI: 10.1016/j.eclinm.2022.101663
Effect of intravenous almitrine on intubation or mortality in patients with COVID-19 acute hypoxemic respiratory failure: A multicentre, randomised, double-blind, placebo-controlled trial
Abstract
Background: Severe hypoxemia in patients with COVID-19 pneumonia might result from hypoxic pulmonary vasoconstriction, contributing to ventilation/perfusion (V/Q) mismatch. Because almitrine improves V/Q, it might reduce the risk for mechanical ventilation (MV) in such patients. Our primary objective was to determine the effect of almitrine on the need for MV at day 7.
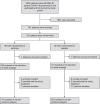
Methods: In a randomised double-blind placebo-controlled trial involving 15 ICUs, patients hospitalized for COVID-19 pneumonia and experiencing acute hypoxemic respiratory failure were randomly assigned to receive 5 days of intravenous low-dose (2 µg.kg-1.min-1) almitrine or placebo. The primary outcome was endotracheal intubation for MV or death within 7 days after randomisation. Secondary outcomes included in-hospital mortality, 28-day mortality, number of ventilator-free days, number of days in the ICU and the hospital, and treatment discontinuation for pre-specified adverse effects. This trial was registered with ClinicalTrials.gov, NCT04357457.
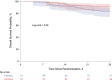
Findings: Between September 3, 2020 and September 25, 2021 181 patients were enrolled and randomly assigned to almitrine (n=89) or placebo (n=92). 179 patients (excluding two who withdrew from the study) were included in the intention-to-treat analysis (mean age: 60·1 years; 34% women) and analyzed. On day 7, the primary endpoint occurred in 32 patients assigned to almitrine (36%) and in 37 patients assigned to placebo (41%), for a difference of -4·3% (95% confidence interval: -18·7% to 10·2%). Secondary outcomes (28-day mortality, in-hospital mortality, ventilator-free days at day 28, days in the ICU and the hospital, and treatment discontinuation for pre-specified adverse effects) did not differ between the two groups.
Interpretation: In patients with COVID-19 acute hypoxemic respiratory failure, low-dose almitrine failed in reducing the need for MV or death at day 7.
Funding: Programme Hospitalier de Recherche Clinique (PHRC COVID 2020) funded by the French Ministry of Health, Les Laboratoires Servier (Suresnes, France) providing the study drug free of charge.
Keywords: ARDS; Almitrine; COVID-19; Hypoxemia; Pneumonia.
© 2022 The Authors.
Conflict of interest statement
PK received personal fees from General Electric Healthcare outside of the submitted work. JFP: Nothing to disclose. AR: Nothing to disclose. BC: Nothing to disclose. MC: Nothing to disclose. AT: Nothing to disclose. JA: Nothing to disclose. FD: personal fees from Sedana medical and Biomerieux; research grant from French Ministry of Health, European Society of Intensive Care Medicine, and Société Française d'Anesthésie Réanimation. JMC: personal fees and non-financial support from Drager, GE Healthcare, Sedana Medical, Baxter, and Amomed; personal fees from Fisher and Paykel Healthcare, Orion, Philips Medical, and Fresenius Medical Care; and nonfinancial support from LFB and Bird Corporation, outside of the submitted work. EW: personal fees from MSD, Akcea therapeutics, and LFB; nonfinancial support from LFB and Akcea therapeutics, outside of the submitted work. FR: Nothing to disclose. YF: Nothing to disclose. TS: research grants or contracts from AstraZeneca, Bayer, Boehringer, Daiichi-Sankyo, Eli-Lilly, GSK, Novartis, and Sanofi; personal fees from Servier, and Novartis; participation on a Data Safety Monitoring Board or Advisory Board from Ablative solutions, Air Liquide, AstraZeneca, Sanofi, Novartis, and 4Living Biotech, outside of the submitted work. BR: Nothing to disclose.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Hydrocortisone plus fludrocortisone for community acquired pneumonia-related septic shock: a subgroup analysis of the APROCCHSS phase 3 randomised trial.Lancet Respir Med. 2024 May;12(5):366-374. doi: 10.1016/S2213-2600(23)00430-7. Epub 2024 Feb 1. Lancet Respir Med. 2024. PMID: 38310918 Clinical Trial.
-
Efficacy and safety of suvratoxumab for prevention of Staphylococcus aureus ventilator-associated pneumonia (SAATELLITE): a multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 2 pilot trial.Lancet Infect Dis. 2021 Sep;21(9):1313-1323. doi: 10.1016/S1473-3099(20)30995-6. Epub 2021 Apr 21. Lancet Infect Dis. 2021. PMID: 33894131 Clinical Trial.
-
Intravenous immunoglobulins in patients with COVID-19-associated moderate-to-severe acute respiratory distress syndrome (ICAR): multicentre, double-blind, placebo-controlled, phase 3 trial.Lancet Respir Med. 2022 Feb;10(2):158-166. doi: 10.1016/S2213-2600(21)00440-9. Epub 2021 Nov 11. Lancet Respir Med. 2022. PMID: 34774185 Free PMC article. Clinical Trial.
-
Simvastatin to reduce pulmonary dysfunction in patients with acute respiratory distress syndrome: the HARP-2 RCT.Southampton (UK): NIHR Journals Library; 2018 Jan. Southampton (UK): NIHR Journals Library; 2018 Jan. PMID: 29400921 Free Books & Documents. Review.
Cited by
-
Use of almitrine in spontaneously breathing patients with COVID-19 treated with high-flow nasal cannula oxygen therapy and with persistent hypoxemia.Respir Res. 2023 Jan 5;24(1):1. doi: 10.1186/s12931-022-02308-y. Respir Res. 2023. PMID: 36600234 Free PMC article.
-
Unraveling the impact of nitric oxide, almitrine, and their combination in COVID-19 (at the edge of sepsis) patients: a systematic review.Front Pharmacol. 2024 Jan 22;14:1172447. doi: 10.3389/fphar.2023.1172447. eCollection 2023. Front Pharmacol. 2024. PMID: 38318311 Free PMC article.
References
-
- IMProving Emergency Care (IMPEC) FHU Collaborators Group. Bouzid D., Visseaux B, Kassasseya C, et al. Comparison of patients infected with Delta versus Omicron COVID-19 variants presenting to Paris emergency departments : a retrospective cohort study. Ann Intern Med. 2022;175:831–837. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

