Determining Toxic Potencies of Water-Soluble Contaminants in Wastewater Influents and Effluent Using Gene Expression Profiling in C. elegans as a Bioanalytical Tool
- PMID: 36190544
- PMCID: PMC9556352
- DOI: 10.1007/s00244-022-00959-y
Determining Toxic Potencies of Water-Soluble Contaminants in Wastewater Influents and Effluent Using Gene Expression Profiling in C. elegans as a Bioanalytical Tool
Abstract
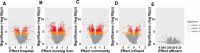
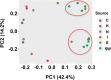
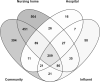
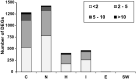
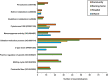
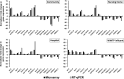
With chemical analysis, it is impossible to qualify and quantify the toxic potency of especially hydrophilic bioactive contaminants. In this study, we applied the nematode C. elegans as a model organism for detecting the toxic potency of whole influent wastewater samples. Gene expression in the nematode was used as bioanalytical tool to reveal the presence, type and potency of molecular pathways induced by 24-h exposure to wastewater from a hospital (H), nursing home (N), community (C), and influent (I) and treated effluent (E) from a local wastewater treatment plant. Exposure to influent water significantly altered expression of 464 genes, while only two genes were differentially expressed in nematodes treated with effluent. This indicates a significant decrease in bioactive pollutant-load after wastewater treatment. Surface water receiving the effluent did not induce any genes in exposed nematodes. A subset of 209 genes was differentially expressed in all untreated wastewaters, including cytochromes P450 and C-type lectins related to the nematode's xenobiotic metabolism and immune response, respectively. Different subsets of genes responded to particular waste streams making them candidates to fingerprint-specific wastewater sources. This study shows that gene expression profiling in C. elegans can be used for mechanism-based identification of hydrophilic bioactive compounds and fingerprinting of specific wastewaters. More comprehensive than with chemical analysis, it can demonstrate the effective overall removal of bioactive compounds through wastewater treatment. This bioanalytical tool can also be applied in the process of identification of the bioactive compounds via a process of toxicity identification evaluation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
A Multiplex Gene Expression Assay for Direct Measurement of RNA Transcripts in Crude Lysates of the Nematode Caenorhabditis elegans Used as a Bioanalytical Tool.Environ Toxicol Chem. 2023 Jan;42(1):130-142. doi: 10.1002/etc.5505. Epub 2022 Dec 1. Environ Toxicol Chem. 2023. PMID: 36282018 Free PMC article.
-
Development of a transcription-based bioanalytical tool to quantify the toxic potencies of hydrophilic compounds in water using the nematode Caenorhabditis elegans.Ecotoxicol Environ Saf. 2021 Dec 20;227:112923. doi: 10.1016/j.ecoenv.2021.112923. Epub 2021 Oct 23. Ecotoxicol Environ Saf. 2021. PMID: 34700171
-
The fate of dissolved organic carbon (DOC) in the wastewater treatment process and its importance in the removal of wastewater contaminants.Environ Sci Pollut Res Int. 2007 Jul;14(5):284-92. doi: 10.1065/espr2006.05.302. Environ Sci Pollut Res Int. 2007. PMID: 17722762
-
Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: current knowledge between 2010 and 2019.Environ Monit Assess. 2022 Mar 30;194(4):306. doi: 10.1007/s10661-022-09846-4. Environ Monit Assess. 2022. PMID: 35353241 Review.
-
The risks associated with wastewater reuse and xenobiotics in the agroecological environment.Sci Total Environ. 2011 Sep 1;409(19):3555-63. doi: 10.1016/j.scitotenv.2010.03.036. Epub 2010 May 1. Sci Total Environ. 2011. PMID: 20435343 Review.
Cited by
-
Using L. minor and C. elegans to assess the ecotoxicity of real-life contaminated soil samples and their remediation by clay- and carbon-based sorbents.Environ Pollut. 2024 Apr 15;347:123762. doi: 10.1016/j.envpol.2024.123762. Epub 2024 Mar 11. Environ Pollut. 2024. PMID: 38479705 Free PMC article.
-
A Multiplex Gene Expression Assay for Direct Measurement of RNA Transcripts in Crude Lysates of the Nematode Caenorhabditis elegans Used as a Bioanalytical Tool.Environ Toxicol Chem. 2023 Jan;42(1):130-142. doi: 10.1002/etc.5505. Epub 2022 Dec 1. Environ Toxicol Chem. 2023. PMID: 36282018 Free PMC article.
References
-
- Escher BI, Neale P, Leusch F (2021) Bioanalytical tools in water quality assessment.10.2166/9781789061987
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

