The precision medicine process for treating rare disease using the artificial intelligence tool mediKanren
- PMID: 36248623
- PMCID: PMC9562701
- DOI: 10.3389/frai.2022.910216
The precision medicine process for treating rare disease using the artificial intelligence tool mediKanren
Abstract
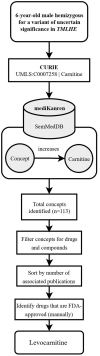
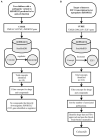
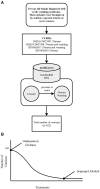
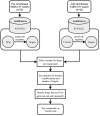
There are over 6,000 different rare diseases estimated to impact 300 million people worldwide. As genetic testing becomes more common practice in the clinical setting, the number of rare disease diagnoses will continue to increase, resulting in the need for novel treatment options. Identifying treatments for these disorders is challenging due to a limited understanding of disease mechanisms, small cohort sizes, interindividual symptom variability, and little commercial incentive to develop new treatments. A promising avenue for treatment is drug repurposing, where FDA-approved drugs are repositioned as novel treatments. However, linking disease mechanisms to drug action can be extraordinarily difficult and requires a depth of knowledge across multiple fields, which is complicated by the rapid pace of biomedical knowledge discovery. To address these challenges, The Hugh Kaul Precision Medicine Institute developed an artificial intelligence tool, mediKanren, that leverages the mechanistic insight of genetic disorders to identify therapeutic options. Using knowledge graphs, mediKanren enables an efficient way to link all relevant literature and databases. This tool has allowed for a scalable process that has been used to help over 500 rare disease families. Here, we provide a description of our process, the advantages of mediKanren, and its impact on rare disease patients.
Keywords: artificial intelligence; biomedical reasoning; drug repurposing; precision medicine; rare disease.
Copyright © 2022 Foksinska, Crowder, Crouse, Henrikson, Byrd, Rosenblatt, Patton, He, Tran-Nguyen, Zheng, Ramsey, Amin, Osborne, UAB Precision Medicine Institute and Might.
Conflict of interest statement
Author JH is employed by Groovescale. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Artificial intelligence in early drug discovery enabling precision medicine.Expert Opin Drug Discov. 2021 Sep;16(9):991-1007. doi: 10.1080/17460441.2021.1918096. Epub 2021 Jun 2. Expert Opin Drug Discov. 2021. PMID: 34075855 Review.
-
Human and Machine Intelligence Together Drive Drug Repurposing in Rare Diseases.Front Genet. 2021 Jul 28;12:707836. doi: 10.3389/fgene.2021.707836. eCollection 2021. Front Genet. 2021. PMID: 34394194 Free PMC article.
-
Contexts and contradictions: a roadmap for computational drug repurposing with knowledge inference.Brief Bioinform. 2022 Jul 18;23(4):bbac268. doi: 10.1093/bib/bbac268. Brief Bioinform. 2022. PMID: 35817308 Free PMC article. Review.
Cited by
-
The Impact of Artificial Intelligence in the Odyssey of Rare Diseases.Biomedicines. 2023 Mar 13;11(3):887. doi: 10.3390/biomedicines11030887. Biomedicines. 2023. PMID: 36979866 Free PMC article. Review.
-
Exploring NCATS in-house biomedical data for evidence-based drug repurposing.PLoS One. 2024 Jan 25;19(1):e0289518. doi: 10.1371/journal.pone.0289518. eCollection 2024. PLoS One. 2024. PMID: 38271343 Free PMC article.
-
Functional genomics and small molecules in mitochondrial neurodevelopmental disorders.Neurotherapeutics. 2024 Jan;21(1):e00316. doi: 10.1016/j.neurot.2024.e00316. Epub 2024 Jan 19. Neurotherapeutics. 2024. PMID: 38244259 Free PMC article. Review.
-
The Impact of Artificial Intelligence on Optimizing Diagnosis and Treatment Plans for Rare Genetic Disorders.Cureus. 2023 Oct 11;15(10):e46860. doi: 10.7759/cureus.46860. eCollection 2023 Oct. Cureus. 2023. PMID: 37954711 Free PMC article. Review.
-
The use of artificial intelligence in the treatment of rare diseases: A scoping review.Intractable Rare Dis Res. 2024 Feb;13(1):12-22. doi: 10.5582/irdr.2023.01111. Intractable Rare Dis Res. 2024. PMID: 38404730 Free PMC article. Review.
References
-
- Cadegiani F. A., McCoy J., Gustavo Wambier C., Vaño-Galván S., Shapiro J., Tosti A., et al. . (2021). Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus 13, e13492. 10.7759/cureus.13492 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

