Systemic therapy for early-stage breast cancer: learning from the past to build the future
- PMID: 36253451
- PMCID: PMC9575647
- DOI: 10.1038/s41571-022-00687-1
Systemic therapy for early-stage breast cancer: learning from the past to build the future
Abstract
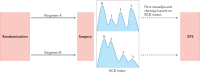
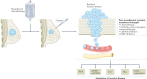
The treatment of breast cancer has improved dramatically over the past century, from a strictly surgical approach to a coordinated one, including local and systemic therapies. Systemic therapies for early-stage disease were initially tested against observation or placebo only in adjuvant trials. Subsequent clinical trials focusing on treatment 'fine-tuning' had a marked increase in cohort size, duration and costs, leading to a growing interest in the neoadjuvant setting in the past decade. Neoadjuvant trial designs have the advantages of enabling the direct evaluation of treatment effects on tumour diameter and offer unique translational research opportunities through the comparative analysis of tumour biology before, during and after treatment. Current technologies enabling the identification of better predictive biomarkers are shaping the new era of (neo)adjuvant trials. An urgent need exists to reinforce collaboration between the pharmaceutical industry and academia to share data and thus establish large databases of biomarker data coupled with patient outcomes that are easily accessible to the scientific community. In this Review, we summarize the evolution of (neo)adjuvant trials from the pre-genomic to the post-genomic era and provide critical insights into how neoadjuvant studies are currently designed, discussing the need for better end points and treatment strategies that are more personalized, including in the post-neoadjuvant setting.
© 2022. Springer Nature Limited.
Conflict of interest statement
E.A. has received fees or honoraria for consultant roles for Eli Lilly and Sandoz and support for attending medical conferences from Eli Lilly, Genetic, Istituto Gentili, Novartis and Roche, all unrelated to the submitted work. J.G. has received fees or honoraria for consultant roles for Daiichi, Eisai, Exact Science, Gilead, Lilly, Merck, Novartis, Onxeo, Pfizer, Roche Genentech and Seattle Genetics, and works in an institution that receives research grants from Eisai, Exact Science and Roche Genentech, all unrelated to the submitted work. M.P. has received fees or honoraria for consultant roles for AstraZeneca, Camel-IDS/Precirix, Frame Therapeutics, Gilead, Immunomedics, Immutep, Lilly, Menarini, MSD, NBE Therapeutics, Novartis, Odonate, Pfizer, Roche Genentech, Seagen and Seattle Genetics, is part of the scientific board at Oncolytics, and works in an institution that receives research grants from AstraZeneca, Immunomedics, Lilly, Menarini, MSD, Novartis, Pfizer, Radius, Roche Genentech, Servier and Synthon, all unrelated to the submitted work.
Figures

Similar articles
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
WSG ADAPT - adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial.Trials. 2013 Aug 19;14:261. doi: 10.1186/1745-6215-14-261. Trials. 2013. PMID: 23958221 Free PMC article. Clinical Trial.
-
Neoadjuvant Therapy for HER2-positive Breast Cancer.Rev Recent Clin Trials. 2017;12(2):81-92. doi: 10.2174/1574887112666170202165049. Rev Recent Clin Trials. 2017. PMID: 28164759 Review.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Evidence reviews for neoadjuvant treatment: Early and locally advanced breast cancer: diagnosis and management: Evidence review J.London: National Institute for Health and Care Excellence (NICE); 2018 Jul. London: National Institute for Health and Care Excellence (NICE); 2018 Jul. PMID: 35072997 Free Books & Documents. Review.
Cited by
-
Subsequent Indications in Oncology Drugs: Pathways, Timelines, and the Inflation Reduction Act.Ther Innov Regul Sci. 2025 Jan;59(1):102-111. doi: 10.1007/s43441-024-00706-6. Epub 2024 Oct 6. Ther Innov Regul Sci. 2025. PMID: 39369117 Free PMC article.
-
CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution?Int J Mol Sci. 2023 Dec 14;24(24):17488. doi: 10.3390/ijms242417488. Int J Mol Sci. 2023. PMID: 38139316 Free PMC article. Review.
-
Inhibition of histone deacetylases attenuates tumor progression and improves immunotherapy in breast cancer.Front Immunol. 2023 Mar 9;14:1164514. doi: 10.3389/fimmu.2023.1164514. eCollection 2023. Front Immunol. 2023. PMID: 36969235 Free PMC article. Review.
-
A prospective study on tumour response assessment methods after neoadjuvant endocrine therapy in early oestrogen receptor-positive breast cancer.Breast Cancer Res. 2024 Jan 3;26(1):3. doi: 10.1186/s13058-023-01756-8. Breast Cancer Res. 2024. PMID: 38173005 Free PMC article.
-
Phenylsulfonimide PPARα Antagonists Enhance Nrf2 Activation and Promote Oxidative Stress-Induced Apoptosis/Pyroptosis in MCF7 Breast Cancer Cells.Int J Mol Sci. 2023 Jan 10;24(2):1316. doi: 10.3390/ijms24021316. Int J Mol Sci. 2023. PMID: 36674831 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

