Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines
- PMID: 36262278
- PMCID: PMC9574042
- DOI: 10.3389/fmed.2022.994160
Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines
Abstract
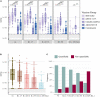
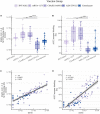
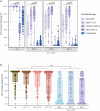
The SARS-CoV-2 pandemic has, as of July 2022, infected more than 550 million people and caused over 6 million deaths across the world. COVID-19 vaccines were quickly developed to protect against severe disease, hospitalization and death. In the present study, we performed a direct comparative analysis of four COVID-19 vaccines: BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), ChAdOx1 (Oxford/AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen), following primary and booster vaccination. We focused on the vaccine-induced antibody-mediated immune response against multiple SARS-CoV-2 variants: wildtype, B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta) and B.1.1.529 (Omicron). The analysis included the quantification of total IgG levels against SARS-CoV-2 Spike, as well as the quantification of antibody neutralization titers. Furthermore, the study assessed the high-throughput ACE2 competition assay as a surrogate for the traditional pseudovirus neutralization assay. The results demonstrated marked differences in antibody-mediated immune responses. The lowest Spike-specific IgG levels and antibody neutralization titers were induced by one dose of the Ad26.COV2.S vaccine, intermediate levels by two doses of the BNT162b2 vaccine, and the highest levels by two doses of the mRNA-1273 vaccine or heterologous vaccination of one dose of the ChAdOx1 vaccine and a subsequent mRNA vaccine. The study also demonstrated that accumulation of SARS-CoV-2 Spike protein mutations was accompanied by a marked decline in antibody neutralization capacity, especially for B.1.1.529. Administration of a booster dose was shown to significantly increase Spike-specific IgG levels and antibody neutralization titers, erasing the differences between the vaccine-induced antibody-mediated immune response between the four vaccines. The findings of this study highlight the importance of booster vaccines and the potential inclusion of future heterologous vaccination strategies for broad protection against current and emerging SARS-CoV-2 variants.
Keywords: COVID-19; SARS-CoV-2; antibodies; booster; immunity; neutralization; omicron; vaccines.
Copyright © 2022 Hvidt, Baerends, Søgaard, Stærke, Raben, Reekie, Nielsen, Johansen, Wiese, Benfield, Iversen, Mustafa, Juhl, Petersen, Ostrowski, Lindvig, Rasmussen, Schleimann, Andersen, Juhl, Dietz, Andreasen, Lundgren, Østergaard, Tolstrup and the ENFORCE Study Group.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared parent affiliation with several of the authors AH, EB, OS, NS, MS, SA, AJ, LD, and SRA at the time of the review.
Figures


Similar articles
-
Comparison of BNT162b2-, mRNA-1273- and Ad26.COV2.S-Elicited IgG and Neutralizing Titers against SARS-CoV-2 and Its Variants.Vaccines (Basel). 2022 May 27;10(6):858. doi: 10.3390/vaccines10060858. Vaccines (Basel). 2022. PMID: 35746466 Free PMC article.
-
Study of efficacy and longevity of immune response to third and fourth doses of COVID-19 vaccines in patients with cancer: A single arm clinical trial.Elife. 2023 Mar 28;12:e83694. doi: 10.7554/eLife.83694. Elife. 2023. PMID: 36975207 Free PMC article. Clinical Trial.
-
Humoral immunity and B-cell memory in response to SARS-CoV-2 infection and vaccination.Biochem Soc Trans. 2022 Dec 16;50(6):1643-1658. doi: 10.1042/BST20220415. Biochem Soc Trans. 2022. PMID: 36421662 Free PMC article.
-
Role of available COVID-19 vaccines in reducing deaths and perspective for next generation vaccines and therapies to counter emerging viral variants: an update.Minerva Med. 2023 Oct;114(5):683-697. doi: 10.23736/S0026-4806.23.08509-9. Epub 2023 Jun 9. Minerva Med. 2023. PMID: 37293890 Review.
-
Vaccinating against a Novel Pathogen: A Critical Review of COVID-19 Vaccine Effectiveness Evidence.Microorganisms. 2023 Dec 31;12(1):89. doi: 10.3390/microorganisms12010089. Microorganisms. 2023. PMID: 38257917 Free PMC article. Review.
Cited by
-
Homologous but not heterologous COVID-19 vaccine booster elicits IgG4+ B-cells and enhanced Omicron subvariant binding.NPJ Vaccines. 2024 Jul 17;9(1):129. doi: 10.1038/s41541-024-00919-8. NPJ Vaccines. 2024. PMID: 39013889 Free PMC article.
-
A 12-month follow-up of the immune response to SARS-CoV-2 primary vaccination: evidence from a real-world study.Front Immunol. 2023 Nov 20;14:1272119. doi: 10.3389/fimmu.2023.1272119. eCollection 2023. Front Immunol. 2023. PMID: 38077369 Free PMC article.
-
Longitudinal Evaluation of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Immunity Over 2 Years Following Vaccination and Infection.J Infect Dis. 2024 Sep 23;230(3):e605-e615. doi: 10.1093/infdis/jiae215. J Infect Dis. 2024. PMID: 38687181 Free PMC article.
-
Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.NPJ Vaccines. 2024 Mar 21;9(1):66. doi: 10.1038/s41541-024-00856-6. NPJ Vaccines. 2024. PMID: 38514656 Free PMC article.
-
Cohort Profile:The Danish National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE).BMJ Open. 2022 Dec 30;12(12):e069065. doi: 10.1136/bmjopen-2022-069065. BMJ Open. 2022. PMID: 36585137 Free PMC article.
References
-
- World Health Organization. WHO Coronavirus (Covid-19) Dashboard. (2022). Available online at: https://covid19.who.int/ (accessed July 12, 2022).
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA Bnt162b2 vaccine against sars-cov-2 infections and covid-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in israel: an observational study using national surveillance data. Lancet. (2021) 397:1819–29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
-
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the chadox1 Ncov-19 vaccine (Azd1222) against sars-cov-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. (2021) 397:99–111. 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

