Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes Ferroptosis-Related Senescence in Adipose Tissue
- PMID: 36297049
- PMCID: PMC9607568
- DOI: 10.3390/nu14204365
Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes Ferroptosis-Related Senescence in Adipose Tissue
Abstract
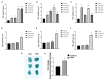
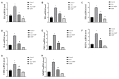
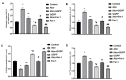
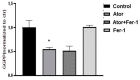
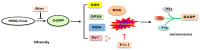
Statin treatment is accepted to prevent adverse cardiovascular events. However, atorvastatin, an HMG-CoA reductase inhibitor, has been reported to exhibit distinct effects on senescent phenotypes. Whether atorvastatin can induce adipose tissue senescence and the mechanisms involved are unknown. The effects of atorvastatin-induced senescence were examined in mouse adipose tissue explants. Here, we showed that statin initiated higher levels of mRNA related to cellular senescence markers and senescence-associated secretory phenotype (SASP), as well as increased accumulation of the senescence-associated β-galactosidase (SA-β-gal) stain in adipose tissues. Furthermore, we found that the levels of reactive oxygen species (ROS), malondialdehyde (MDA), and Fe2+ were elevated in adipose tissues treated with atorvastatin, accompanied by a decrease in the expression of glutathione (GSH), and glutathione peroxidase 4 (GPX4), indicating an iron-dependent ferroptosis. Atorvastatin-induced was prevented by a selective ferroptosis inhibitor (Fer-1). Moreover, supplementation with geranylgeranyl pyrophosphate (GGPP), a metabolic intermediate, reversed atorvastatin-induced senescence, SASP, and lipid peroxidation in adipose tissue explants. Atorvastatin depleted GGPP production, but not Fer-1. Atorvastatin was able to induce ferroptosis in adipose tissue, which was due to increased ROS and an increase in cellular senescence. Moreover, this effect could be reversed by the supplement of GGPP. Taken together, our results suggest that the induction of ferroptosis contributed to statin-induced cell senescence in adipose tissue.
Keywords: adipose tissue; ferroptosis; obesity; senescence; statin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9-Dependent Adipose Insulin Resistance.Nutrients. 2022 Dec 14;14(24):5314. doi: 10.3390/nu14245314. Nutrients. 2022. PMID: 36558473 Free PMC article.
-
Lipid Peroxidation, GSH Depletion, and SLC7A11 Inhibition Are Common Causes of EMT and Ferroptosis in A549 Cells, but Different in Specific Mechanisms.DNA Cell Biol. 2021 Feb;40(2):172-183. doi: 10.1089/dna.2020.5730. Epub 2020 Dec 22. DNA Cell Biol. 2021. PMID: 33351681
-
Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells.Cell Death Dis. 2018 Jul 9;9(7):753. doi: 10.1038/s41419-018-0794-4. Cell Death Dis. 2018. PMID: 29988039 Free PMC article.
-
GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis.Proteomics. 2019 Sep;19(18):e1800311. doi: 10.1002/pmic.201800311. Epub 2019 May 31. Proteomics. 2019. PMID: 30888116 Review.
-
Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy?Metabolism. 2023 Sep;146:155659. doi: 10.1016/j.metabol.2023.155659. Epub 2023 Jul 11. Metabolism. 2023. PMID: 37442270 Review.
Cited by
-
Iron deposition in gastric black spots: Clinicopathological insights and NanoSuit-correlative light and electron microscopy analysis.DEN Open. 2024 Jun 17;5(1):e398. doi: 10.1002/deo2.398. eCollection 2025 Apr. DEN Open. 2024. PMID: 38895560 Free PMC article.
-
Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes PCSK9-Dependent Adipose Insulin Resistance.Nutrients. 2022 Dec 14;14(24):5314. doi: 10.3390/nu14245314. Nutrients. 2022. PMID: 36558473 Free PMC article.
-
The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients.Curr Issues Mol Biol. 2023 Apr 5;45(4):3146-3167. doi: 10.3390/cimb45040205. Curr Issues Mol Biol. 2023. PMID: 37185729 Free PMC article. Review.
-
Ferroptosis: a new hunter of hepatocellular carcinoma.Cell Death Discov. 2024 Mar 13;10(1):136. doi: 10.1038/s41420-024-01863-1. Cell Death Discov. 2024. PMID: 38480712 Free PMC article. Review.
-
The Emerging Roles of Ferroptosis in Pathophysiology and Treatment of Acute Lung Injury.J Inflamm Res. 2023 Sep 14;16:4073-4085. doi: 10.2147/JIR.S420676. eCollection 2023. J Inflamm Res. 2023. PMID: 37727372 Free PMC article. Review.
References
-
- Volonte D., Zou H., Bartholomew J.N., Liu Z., Morel P.A., Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes P53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) J. Biol. Chem. 2015;290:4202–4214. doi: 10.1074/jbc.M114.598268. - DOI - PMC - PubMed
-
- Rezaie-Majd A., Maca T., Bucek R.A., Valent P., Müller M.R., Husslein P., Kashanipour A., Minar E., Baghestanian M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arter. Thromb. Vasc. Biol. 2002;22:1194–1199. doi: 10.1161/01.ATV.0000022694.16328.CC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

