Improvement of non-invasive tests of liver steatosis and fibrosis as indicators for non-alcoholic fatty liver disease in type 2 diabetes mellitus patients with elevated cardiovascular risk profile using the PPAR-α/γ agonist aleglitazar
- PMID: 36378671
- PMCID: PMC9665379
- DOI: 10.1371/journal.pone.0277706
Improvement of non-invasive tests of liver steatosis and fibrosis as indicators for non-alcoholic fatty liver disease in type 2 diabetes mellitus patients with elevated cardiovascular risk profile using the PPAR-α/γ agonist aleglitazar
Abstract
Background: Peroxisome proliferator-activated receptor (PPAR) agonists may have favorable outcomes on non-alcoholic fatty liver disease. This study serves as proof of concept to evaluate whether dual PPAR-α/γ agonists improve non-invasive tests of liver steatosis and fibrosis.
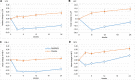
Methods: This is a post-hoc analysis of a randomized, double-blind, placebo-controlled, multi-center trial comprising 7226 patients with type 2 diabetes mellitus and recent coronary artery disease randomized to receive aleglitazar, a PPAR-α/γ agonists, or placebo for two years. Main outcomes were change in non-invasive tests for liver steatosis and fibrosis: Liver Fat Score (LFS), Liver Accumulation Product (LAP), Fibrosis-4 (FIB-4), and NAFLD Fibrosis Score (NFS).
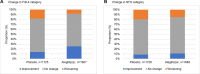
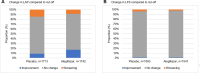
Results: LFS, LAP and FIB-4 decreased upon treatment, whereas scores in the placebo group remained the same or increased (P<0.001). NFS responded differently but remained consistently lower than placebo. In the treatment group more participants shifted to a lower FIB-4 and NFS category, or improved in respect to the LAP cut-off values compared to the placebo group (P<0.001 for FIB-4 and LAP, P<0.004 for NFS). LFS had a low discriminative power in this study.
Conclusion: This post-hoc analysis showed improvement of non-invasive tests of liver steatosis and fibrosis after starting dual PPAR-α/γ agonist treatment, adding to the evidence that this pathway has potential in non-alcoholic fatty liver disease treatment.
Copyright: © 2022 Grobbee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study.Lancet. 2009 Jul 11;374(9684):126-35. doi: 10.1016/S0140-6736(09)60870-9. Epub 2009 Jun 8. Lancet. 2009. PMID: 19515415 Clinical Trial.
-
Non-Alcoholic Fatty Liver Disease Treatment in Patients with Type 2 Diabetes Mellitus; New Kids on the Block.Curr Vasc Pharmacol. 2020;18(2):172-181. doi: 10.2174/1570161117666190405164313. Curr Vasc Pharmacol. 2020. PMID: 30961499 Review.
-
Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening.Gastroenterology. 2016 May;150(5):1147-1159.e5. doi: 10.1053/j.gastro.2016.01.038. Epub 2016 Feb 11. Gastroenterology. 2016. PMID: 26874076 Clinical Trial.
-
Therapeutic potential of aleglitazar, a new dual PPAR-α/γ agonist: implications for cardiovascular disease in patients with diabetes mellitus.Am J Cardiovasc Drugs. 2010;10(4):209-16. doi: 10.2165/11539500-000000000-00000. Am J Cardiovasc Drugs. 2010. PMID: 20653327
-
New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence.Cardiovasc Diabetol. 2019 Jun 17;18(1):80. doi: 10.1186/s12933-019-0884-3. Cardiovasc Diabetol. 2019. PMID: 31208414 Free PMC article. Review.
Cited by
-
Non-Alcoholic Fatty Liver Disease: Translating Disease Mechanisms into Therapeutics Using Animal Models.Int J Mol Sci. 2023 Jun 10;24(12):9996. doi: 10.3390/ijms24129996. Int J Mol Sci. 2023. PMID: 37373143 Free PMC article. Review.
-
Signaling pathways in macrophages: molecular mechanisms and therapeutic _targets.MedComm (2020). 2023 Sep 11;4(5):e349. doi: 10.1002/mco2.349. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37706196 Free PMC article. Review.
-
Mechanism of Metabolic Dysfunction-associated Steatotic Liver Disease: Important role of lipid metabolism.J Clin Transl Hepatol. 2024 Sep 28;12(9):815-826. doi: 10.14218/JCTH.2024.00019. Epub 2024 Sep 3. J Clin Transl Hepatol. 2024. PMID: 39280069 Free PMC article. Review.
References
-
- Duell PB, Welty F. K., Miller M., Chait A., Hammond G., Ahmad Z, et al.. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arteriosclerosis, Thrombosis, and Vascular Biology. 2022;42(6):168–e85. doi: 10.1161/ATV.0000000000000153 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

