Retinoic acid-induced 1 gene haploinsufficiency alters lipid metabolism and causes autophagy defects in Smith-Magenis syndrome
- PMID: 36411275
- PMCID: PMC9678881
- DOI: 10.1038/s41419-022-05410-7
Retinoic acid-induced 1 gene haploinsufficiency alters lipid metabolism and causes autophagy defects in Smith-Magenis syndrome
Abstract
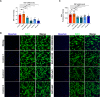
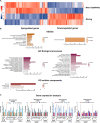
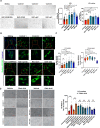
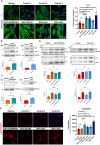
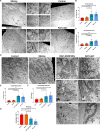
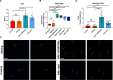
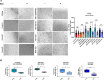
Smith-Magenis syndrome (SMS) is a neurodevelopmental disorder characterized by cognitive and behavioral symptoms, obesity, and sleep disturbance, and no therapy has been developed to alleviate its symptoms or delay disease onset. SMS occurs due to haploinsufficiency of the retinoic acid-induced-1 (RAI1) gene caused by either chromosomal deletion (SMS-del) or RAI1 missense/nonsense mutation. The molecular mechanisms underlying SMS are unknown. Here, we generated and characterized primary cells derived from four SMS patients (two with SMS-del and two carrying RAI1 point mutations) and four control subjects to investigate the pathogenetic processes underlying SMS. By combining transcriptomic and lipidomic analyses, we found altered expression of lipid and lysosomal genes, deregulation of lipid metabolism, accumulation of lipid droplets, and blocked autophagic flux. We also found that SMS cells exhibited increased cell death associated with the mitochondrial pathology and the production of reactive oxygen species. Treatment with N-acetylcysteine reduced cell death and lipid accumulation, which suggests a causative link between metabolic dyshomeostasis and cell viability. Our results highlight the pathological processes in human SMS cells involving lipid metabolism, autophagy defects and mitochondrial dysfunction and suggest new potential therapeutic _targets for patient treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Smith-Magenis syndrome: haploinsufficiency of RAI1 results in altered gene regulation in neurological and metabolic pathways.Expert Rev Mol Med. 2011 Apr 19;13:e14. doi: 10.1017/S1462399411001827. Expert Rev Mol Med. 2011. PMID: 21545756 Review.
-
Molecular analysis of the Retinoic Acid Induced 1 gene (RAI1) in patients with suspected Smith-Magenis syndrome without the 17p11.2 deletion.PLoS One. 2011;6(8):e22861. doi: 10.1371/journal.pone.0022861. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21857958 Free PMC article.
-
RAI1 transcription factor activity is impaired in mutants associated with Smith-Magenis Syndrome.PLoS One. 2012;7(9):e45155. doi: 10.1371/journal.pone.0045155. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028815 Free PMC article.
-
Smith-Magenis syndrome results in disruption of CLOCK gene transcription and reveals an integral role for RAI1 in the maintenance of circadian rhythmicity.Am J Hum Genet. 2012 Jun 8;90(6):941-9. doi: 10.1016/j.ajhg.2012.04.013. Epub 2012 May 10. Am J Hum Genet. 2012. PMID: 22578325 Free PMC article.
-
Smith-Magenis Syndrome-Clinical Review, Biological Background and Related Disorders.Genes (Basel). 2022 Feb 11;13(2):335. doi: 10.3390/genes13020335. Genes (Basel). 2022. PMID: 35205380 Free PMC article. Review.
Cited by
-
Clarifying main nutritional aspects and resting energy expenditure in children with Smith-Magenis syndrome.Eur J Pediatr. 2024 Oct;183(10):4563-4571. doi: 10.1007/s00431-024-05715-z. Epub 2024 Aug 20. Eur J Pediatr. 2024. PMID: 39162735 Free PMC article.
-
Dystonia: A novel sign of the Smith-Magenis syndrome - A three-case report.Clin Park Relat Disord. 2024 Aug 6;11:100267. doi: 10.1016/j.prdoa.2024.100267. eCollection 2024. Clin Park Relat Disord. 2024. PMID: 39224875 Free PMC article. No abstract available.
-
Metabolic Profile of Patients with Smith-Magenis Syndrome: An Observational Study with Literature Review.Children (Basel). 2023 Aug 25;10(9):1451. doi: 10.3390/children10091451. Children (Basel). 2023. PMID: 37761412 Free PMC article.
-
A case of Smith-Magenis syndrome with skin manifestations caused by a novel locus mutation in the RAI1 gene.J Int Med Res. 2023 Sep;51(9):3000605231190553. doi: 10.1177/03000605231190553. J Int Med Res. 2023. PMID: 37756600 Free PMC article.
References
-
- Smith ACM, Boyd KE, Brennan C, Charles J, Elsea SH, Finucane BM, et al. Smith-Magenis Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW et al. (eds). GeneReviews. University of Washington, Seattle: Seattle (WA), 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

