Humoral immunity and B-cell memory in response to SARS-CoV-2 infection and vaccination
- PMID: 36421662
- PMCID: PMC9788580
- DOI: 10.1042/BST20220415
Humoral immunity and B-cell memory in response to SARS-CoV-2 infection and vaccination
Abstract
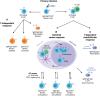
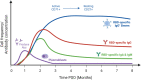
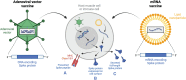
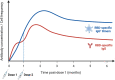
Natural infection with SARS-CoV-2 induces a robust circulating memory B cell (Bmem) population, which remains stable in number at least 8 months post-infection despite the contraction of antibody levels after 1 month. Multiple vaccines have been developed to combat the virus. These include two new formulations, mRNA and adenoviral vector vaccines, which have varying efficacy rates, potentially related to their distinct capacities to induce humoral immune responses. The mRNA vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) elicit significantly higher serum IgG and neutralizing antibody levels than the adenoviral vector ChAdOx1 (AstraZeneca) and Ad26.COV2.S (Janssen) vaccines. However, all vaccines induce Spike- and RBD-specific Bmem, which are vital in providing long-lasting protection in the form of rapid recall responses to subsequent infections. Past and current SARS-CoV-2 variants of concern (VoC) have shown the capacity to escape antibody neutralization to varying degrees. A booster dose with an mRNA vaccine following primary vaccination restores antibody levels and improves the capacity of these antibodies and Bmem to bind viral variants, including the current VoC Omicron. Future experimental research will be essential to evaluate the durability of protection against VoC provided by each vaccine and to identify immune markers of protection to enable prognostication of people who are at risk of severe complications from COVID-19.
Keywords: COVID-19; IgG; Omicron; SARS-CoV-2; memory B cell; neutralizing antibody.
© 2022 The Author(s).
Conflict of interest statement
M.C.v.Z. and R.O.H. are inventors on a patent application related to generation of fluorescently labeled antigen tetramers used for flow cytometric analysis of antigen-specific B cells. The other authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
COVID-19 Adenoviral Vector Vaccination Elicits a Robust Memory B Cell Response with the Capacity to Recognize Omicron BA.2 and BA.5 Variants.J Clin Immunol. 2023 Oct;43(7):1506-1518. doi: 10.1007/s10875-023-01527-2. Epub 2023 Jun 16. J Clin Immunol. 2023. PMID: 37322095 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection.Front Immunol. 2023 Mar 7;14:1131229. doi: 10.3389/fimmu.2023.1131229. eCollection 2023. Front Immunol. 2023. PMID: 36960070 Free PMC article.
-
Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
-
Role of COVID-19 Vaccines in SARS-CoV-2 Variants.Front Immunol. 2022 May 20;13:898192. doi: 10.3389/fimmu.2022.898192. eCollection 2022. Front Immunol. 2022. PMID: 35669787 Free PMC article. Review.
Cited by
-
An Overview of the Strategies to Boost SARS-CoV-2-Specific Immunity in People with Inborn Errors of Immunity.Vaccines (Basel). 2024 Jun 18;12(6):675. doi: 10.3390/vaccines12060675. Vaccines (Basel). 2024. PMID: 38932404 Free PMC article. Review.
-
OVX033, a nucleocapsid-based vaccine candidate, provides broad-spectrum protection against SARS-CoV-2 variants in a hamster challenge model.Front Immunol. 2023 Jun 19;14:1188605. doi: 10.3389/fimmu.2023.1188605. eCollection 2023. Front Immunol. 2023. PMID: 37409116 Free PMC article.
-
Evaluation of the Abdala Vaccine: Antibody and Cellular Response to the RBD Domain of SARS-CoV-2.Vaccines (Basel). 2023 Nov 30;11(12):1787. doi: 10.3390/vaccines11121787. Vaccines (Basel). 2023. PMID: 38140191 Free PMC article.
-
A comprehensive perspective on the interaction between gut microbiota and COVID-19 vaccines.Gut Microbes. 2023 Jan-Dec;15(1):2233146. doi: 10.1080/19490976.2023.2233146. Gut Microbes. 2023. PMID: 37431857 Free PMC article. Review.
-
Homologous but not heterologous COVID-19 vaccine booster elicits IgG4+ B-cells and enhanced Omicron subvariant binding.NPJ Vaccines. 2024 Jul 17;9(1):129. doi: 10.1038/s41541-024-00919-8. NPJ Vaccines. 2024. PMID: 39013889 Free PMC article.
References
-
- World Health Organisation. WHO Coronavirus (COVID-19) Dashboard 2022. Available from: https://covid19.who.int/
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

