_targeting the Complement-Sphingolipid System in COVID-19 and Gaucher Diseases: Evidence for a New Treatment Strategy
- PMID: 36430817
- PMCID: PMC9695449
- DOI: 10.3390/ijms232214340
_targeting the Complement-Sphingolipid System in COVID-19 and Gaucher Diseases: Evidence for a New Treatment Strategy
Abstract
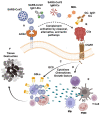
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)-induced disease (COVID-19) and Gaucher disease (GD) exhibit upregulation of complement 5a (C5a) and its C5aR1 receptor, and excess synthesis of glycosphingolipids that lead to increased infiltration and activation of innate and adaptive immune cells, resulting in massive generation of pro-inflammatory cytokines, chemokines and growth factors. This C5a-C5aR1-glycosphingolipid pathway- induced pro-inflammatory environment causes the tissue damage in COVID-19 and GD. Strikingly, pharmaceutically _targeting the C5a-C5aR1 axis or the glycosphingolipid synthesis pathway led to a reduction in glycosphingolipid synthesis and innate and adaptive immune inflammation, and protection from the tissue destruction in both COVID-19 and GD. These results reveal a common involvement of the complement and glycosphingolipid systems driving immune inflammation and tissue damage in COVID-19 and GD, respectively. It is therefore expected that combined _targeting of the complement and sphingolipid pathways could ameliorate the tissue destruction, organ failure, and death in patients at high-risk of developing severe cases of COVID-19.
Keywords: inflammation; innate and adaptive immunity; lipid; rare-genetic disease; viral infection.
Conflict of interest statement
Author affirms that that this manuscript was completed in absence of any commercial or financial interactions that could be interpreted as a potential conflict of interest.
Figures
Similar articles
-
C5a Activates a Pro-Inflammatory Gene Expression Profile in Human Gaucher iPSC-Derived Macrophages.Int J Mol Sci. 2021 Sep 14;22(18):9912. doi: 10.3390/ijms22189912. Int J Mol Sci. 2021. PMID: 34576075 Free PMC article.
-
Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease.Nature. 2017 Mar 2;543(7643):108-112. doi: 10.1038/nature21368. Epub 2017 Feb 22. Nature. 2017. PMID: 28225753
-
C5aR1 signaling triggers lung immunopathology in COVID-19 through neutrophil extracellular traps.J Clin Invest. 2023 Jun 15;133(12):e163105. doi: 10.1172/JCI163105. J Clin Invest. 2023. PMID: 37104043 Free PMC article.
-
An unexpected player in Gaucher disease: The multiple roles of complement in disease development.Semin Immunol. 2018 Jun;37:30-42. doi: 10.1016/j.smim.2018.02.006. Epub 2018 Feb 23. Semin Immunol. 2018. PMID: 29478824 Review.
-
[Involvement of the complement cascade in severe forms of COVID-19].Med Sci (Paris). 2021 Apr;37(4):333-341. doi: 10.1051/medsci/2021021. Epub 2021 Apr 9. Med Sci (Paris). 2021. PMID: 33835019 Review. French.
Cited by
-
Exploring Pro-Inflammatory Immunological Mediators: Unraveling the Mechanisms of Neuroinflammation in Lysosomal Storage Diseases.Biomedicines. 2023 Apr 1;11(4):1067. doi: 10.3390/biomedicines11041067. Biomedicines. 2023. PMID: 37189685 Free PMC article. Review.
-
COVID-19 and neurological disorders: what might connect Parkinson's disease to SARS-CoV-2 infection.Front Neurol. 2023 May 18;14:1172416. doi: 10.3389/fneur.2023.1172416. eCollection 2023. Front Neurol. 2023. PMID: 37273689 Free PMC article. Review.
-
Inflammation in Fabry disease: stages, molecular pathways, and therapeutic implications.Front Cardiovasc Med. 2024 Jun 12;11:1420067. doi: 10.3389/fcvm.2024.1420067. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38932991 Free PMC article. Review.
-
The metaproteome of the gut microbiota in pediatric patients affected by COVID-19.Front Cell Infect Microbiol. 2023 Dec 22;13:1327889. doi: 10.3389/fcimb.2023.1327889. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38188629 Free PMC article.
-
Development and validation of a novel CD4+ T cell-related gene signature to detect severe COVID-19.Clin Transl Med. 2023 Jun;13(6):e1294. doi: 10.1002/ctm2.1294. Clin Transl Med. 2023. PMID: 37278129 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

