Propranolol Modulates Cerebellar Circuit Activity and Reduces Tremor
- PMID: 36497147
- PMCID: PMC9740691
- DOI: 10.3390/cells11233889
Propranolol Modulates Cerebellar Circuit Activity and Reduces Tremor
Abstract
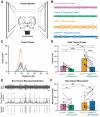
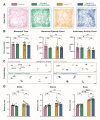
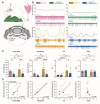
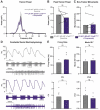
Tremor is the most common movement disorder. Several drugs reduce tremor severity, but no cures are available. Propranolol, a β-adrenergic receptor blocker, is the leading treatment for tremor. However, the in vivo circuit mechanisms by which propranolol decreases tremor remain unclear. Here, we test whether propranolol modulates activity in the cerebellum, a key node in the tremor network. We investigated the effects of propranolol in healthy control mice and Car8wdl/wdl mice, which exhibit pathophysiological tremor and ataxia due to cerebellar dysfunction. Propranolol reduced physiological tremor in control mice and reduced pathophysiological tremor in Car8wdl/wdl mice to control levels. Open field and footprinting assays showed that propranolol did not correct ataxia in Car8wdl/wdl mice. In vivo recordings in awake mice revealed that propranolol modulates the spiking activity of control and Car8wdl/wdl Purkinje cells. Recordings in cerebellar nuclei neurons, the _targets of Purkinje cells, also revealed altered activity in propranolol-treated control and Car8wdl/wdl mice. Next, we tested whether propranolol reduces tremor through β1 and β2 adrenergic receptors. Propranolol did not change tremor amplitude or cerebellar nuclei activity in β1 and β2 null mice or Car8wdl/wdl mice lacking β1 and β2 receptor function. These data show that propranolol can modulate cerebellar circuit activity through β-adrenergic receptors and may contribute to tremor therapeutics.
Keywords: beta-adrenergic receptors; cerebellum; circuitry; electrophysiology; propranolol; tremor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Persistent motor dysfunction despite homeostatic rescue of cerebellar morphogenesis in the Car8 waddles mutant mouse.Neural Dev. 2019 Mar 12;14(1):6. doi: 10.1186/s13064-019-0130-4. Neural Dev. 2019. PMID: 30867000 Free PMC article.
-
Climbing Fiber Development Is Impaired in Postnatal Car8 wdl Mice.Cerebellum. 2018 Feb;17(1):56-61. doi: 10.1007/s12311-017-0886-1. Cerebellum. 2018. PMID: 28940157 Free PMC article.
-
Pathogenesis of severe ataxia and tremor without the typical signs of neurodegeneration.Neurobiol Dis. 2016 Feb;86:86-98. doi: 10.1016/j.nbd.2015.11.008. Epub 2015 Nov 14. Neurobiol Dis. 2016. PMID: 26586559 Free PMC article.
-
Calcium Signaling, PKC Gamma, IP3R1 and CAR8 Link Spinocerebellar Ataxias and Purkinje Cell Dendritic Development.Curr Neuropharmacol. 2018 Jan 30;16(2):151-159. doi: 10.2174/1570159X15666170529104000. Curr Neuropharmacol. 2018. PMID: 28554312 Free PMC article. Review.
-
Survival strategies for mouse cerebellar Purkinje neurons lacking PMCA2.Neurosci Lett. 2018 Jan 10;663:25-28. doi: 10.1016/j.neulet.2017.09.045. Neurosci Lett. 2018. PMID: 29452612 Review.
Cited by
-
Cerebellar nuclei cells produce distinct pathogenic spike signatures in mouse models of ataxia, dystonia, and tremor.Elife. 2024 Jul 29;12:RP91483. doi: 10.7554/eLife.91483. Elife. 2024. PMID: 39072369 Free PMC article.
-
Cerebellar dysfunction in rodent models with dystonia, tremor, and ataxia.Dystonia. 2023;2:11515. doi: 10.3389/dyst.2023.11515. Epub 2023 Dec 8. Dystonia. 2023. PMID: 38105800 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

