Cochlear resident macrophage mediates development of ribbon synapses via CX3CR1/CX3CL1 axis
- PMID: 36518186
- PMCID: PMC9742371
- DOI: 10.3389/fnmol.2022.1031278
Cochlear resident macrophage mediates development of ribbon synapses via CX3CR1/CX3CL1 axis
Abstract
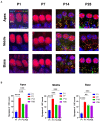
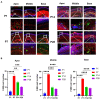
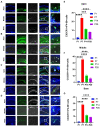
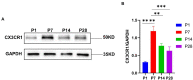
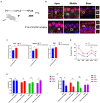
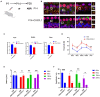
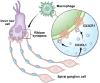
Cochlear ribbon synapses formed between spiral ganglion neurons and inner hair cells in postnatal mice must undergo significant morphological and functional development to reach auditory maturation. However, the mechanisms underlying cochlear ribbon synapse remodeling remain unclear. This study found that cochlear resident macrophages are essential for cochlear ribbon synapse development and maturation in mice via the CX3CR1/CX3CL1 axis. CX3CR1 expression (a macrophage surface-specific receptor) and macrophage count in the cochlea were significantly increased from postnatal day 7 then decreased from days 14 to 28. Seven-day treatment with CX3CR1 inhibitors and artificial upregulation of CX3CL1 levels in the inner ear environment using the semicircular canal injection technique were initiated on day 7, and this resulted in a significant increase in hearing threshold on day 28. Additionally, abnormalities in the morphology and number of cochlear ribbon synapses were detected on day P14, which may be associated with hearing impairment. In conclusion, macrophage regulation of cochlear ribbon synapse remodeling via the CX3CR1/CX3CL1 axis is required during hearing development and offers a new perspective on immune-related hearing loss throughout auditory development. Importantly, it could be a new treatment _target for sensorineural hearing loss.
Keywords: CX3CR1/CX3CL1; cochlear macrophage; development; hearing Loss; ribbon synapses.
Copyright © 2022 Song, Li, Guo, Yu, Liu, Teng, Chen, Xie, Gong and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Autophagy is Required for Remodeling in Postnatal Developing Ribbon Synapses of Cochlear Inner Hair Cells.Neuroscience. 2020 Apr 1;431:1-16. doi: 10.1016/j.neuroscience.2020.01.032. Epub 2020 Feb 4. Neuroscience. 2020. PMID: 32032574
-
Local delivery of soluble fractalkine (CX3CL1) peptide restores ribbon synapses after noise-induced cochlear synaptopathy.Front Cell Neurosci. 2024 Oct 30;18:1486740. doi: 10.3389/fncel.2024.1486740. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39539341 Free PMC article.
-
Macrophages Are Dispensable for Postnatal Pruning of the Cochlear Ribbon Synapses.Front Cell Neurosci. 2021 Oct 21;15:736120. doi: 10.3389/fncel.2021.736120. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34744631 Free PMC article.
-
Innate Immunity to Spiral Ganglion Neuron Loss: A Neuroprotective Role of Fractalkine Signaling in Injured Cochlea.Front Cell Neurosci. 2021 Aug 2;15:694292. doi: 10.3389/fncel.2021.694292. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34408629 Free PMC article. Review.
-
Current concepts in cochlear ribbon synapse formation.Synapse. 2019 May;73(5):e22087. doi: 10.1002/syn.22087. Epub 2019 Feb 18. Synapse. 2019. PMID: 30592086 Free PMC article. Review.
Cited by
-
High doses of radiation cause cochlear immunological stress and sensorineural hearing loss.Heliyon. 2024 Aug 31;10(18):e37223. doi: 10.1016/j.heliyon.2024.e37223. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309931 Free PMC article.
-
Mammalian Inner Ear-Resident Immune Cells-A Scoping Review.Cells. 2024 Sep 12;13(18):1528. doi: 10.3390/cells13181528. Cells. 2024. PMID: 39329712 Free PMC article. Review.
-
Complement factor B is essential for the proper function of the peripheral auditory system.Front Neurol. 2023 Jul 25;14:1214408. doi: 10.3389/fneur.2023.1214408. eCollection 2023. Front Neurol. 2023. PMID: 37560455 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

