Deep-Learning-Aided Diagnosis of Diabetic Retinopathy, Age-Related Macular Degeneration, and Glaucoma Based on Structural and Angiographic OCT
- PMID: 36579336
- PMCID: PMC9791595
- DOI: 10.1016/j.xops.2022.100245
Deep-Learning-Aided Diagnosis of Diabetic Retinopathy, Age-Related Macular Degeneration, and Glaucoma Based on Structural and Angiographic OCT
Abstract
Purpose: Timely diagnosis of eye diseases is paramount to obtaining the best treatment outcomes. OCT and OCT angiography (OCTA) have several advantages that lend themselves to early detection of ocular pathology; furthermore, the techniques produce large, feature-rich data volumes. However, the full clinical potential of both OCT and OCTA is stymied when complex data acquired using the techniques must be manually processed. Here, we propose an automated diagnostic framework based on structural OCT and OCTA data volumes that could substantially support the clinical application of these technologies.
Design: Cross sectional study.
Participants: Five hundred twenty-six OCT and OCTA volumes were scanned from the eyes of 91 healthy participants, 161 patients with diabetic retinopathy (DR), 95 patients with age-related macular degeneration (AMD), and 108 patients with glaucoma.
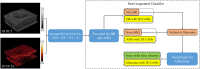
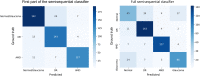
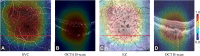
Methods: The diagnosis framework was constructed based on semisequential 3-dimensional (3D) convolutional neural networks. The trained framework classifies combined structural OCT and OCTA scans as normal, DR, AMD, or glaucoma. Fivefold cross-validation was performed, with 60% of the data reserved for training, 20% for validation, and 20% for testing. The training, validation, and test data sets were independent, with no shared patients. For scans diagnosed as DR, AMD, or glaucoma, 3D class activation maps were generated to highlight subregions that were considered important by the framework for automated diagnosis.
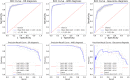
Main outcome measures: The area under the curve (AUC) of the receiver operating characteristic curve and quadratic-weighted kappa were used to quantify the diagnostic performance of the framework.
Results: For the diagnosis of DR, the framework achieved an AUC of 0.95 ± 0.01. For the diagnosis of AMD, the framework achieved an AUC of 0.98 ± 0.01. For the diagnosis of glaucoma, the framework achieved an AUC of 0.91 ± 0.02.
Conclusions: Deep learning frameworks can provide reliable, sensitive, interpretable, and fully automated diagnosis of eye diseases.
Financial disclosures: Proprietary or commercial disclosure may be found after the references.
Keywords: 3D, 3-dimensional; AMD, age-related macular degeneration; AUC, area under the curve; Age-related macular degeneration; CAM, class activation map; DR, diabetic retinopathy; Deep learning; Diabetic retinopathy; Glaucoma; OCT; OCTA, OCT angiography; ROC, receiver operating characteristic.
© 2022 Published by Elsevier Inc. on behalf of American Academy of Ophthalmology.
Figures


Similar articles
-
A Diabetic Retinopathy Classification Framework Based on Deep-Learning Analysis of OCT Angiography.Transl Vis Sci Technol. 2022 Jul 8;11(7):10. doi: 10.1167/tvst.11.7.10. Transl Vis Sci Technol. 2022. PMID: 35822949 Free PMC article.
-
Radiomics-Based Assessment of OCT Angiography Images for Diabetic Retinopathy Diagnosis.Ophthalmol Sci. 2022 Nov 21;3(2):100259. doi: 10.1016/j.xops.2022.100259. eCollection 2023 Jun. Ophthalmol Sci. 2022. PMID: 36578904 Free PMC article.
-
Quantification of Nonperfusion Area in Montaged Widefield OCT Angiography Using Deep Learning in Diabetic Retinopathy.Ophthalmol Sci. 2021 May 12;1(2):100027. doi: 10.1016/j.xops.2021.100027. eCollection 2021 Jun. Ophthalmol Sci. 2021. PMID: 36249293 Free PMC article.
-
Promising Artificial Intelligence-Machine Learning-Deep Learning Algorithms in Ophthalmology.Asia Pac J Ophthalmol (Phila). 2019 May-Jun;8(3):264-272. doi: 10.22608/APO.2018479. Epub 2019 May 31. Asia Pac J Ophthalmol (Phila). 2019. PMID: 31149787 Review.
-
OCT and OCT Angiography Update: Clinical Application to Age-Related Macular Degeneration, Central Serous Chorioretinopathy, Macular Telangiectasia, and Diabetic Retinopathy.Diagnostics (Basel). 2023 Jan 8;13(2):232. doi: 10.3390/diagnostics13020232. Diagnostics (Basel). 2023. PMID: 36673042 Free PMC article. Review.
Cited by
-
Deep-Ocular: Improved Transfer Learning Architecture Using Self-Attention and Dense Layers for Recognition of Ocular Diseases.Diagnostics (Basel). 2023 Oct 10;13(20):3165. doi: 10.3390/diagnostics13203165. Diagnostics (Basel). 2023. PMID: 37891986 Free PMC article.
-
Advancing Ocular Imaging: A Hybrid Attention Mechanism-Based U-Net Model for Precise Segmentation of Sub-Retinal Layers in OCT Images.Bioengineering (Basel). 2024 Feb 28;11(3):240. doi: 10.3390/bioengineering11030240. Bioengineering (Basel). 2024. PMID: 38534514 Free PMC article.
-
AI-based 3D analysis of retinal vasculature associated with retinal diseases using OCT angiography.Biomed Opt Express. 2024 Oct 17;15(11):6416-6432. doi: 10.1364/BOE.534703. eCollection 2024 Nov 1. Biomed Opt Express. 2024. PMID: 39553857 Free PMC article.
-
Artificial intelligence in glaucoma: opportunities, challenges, and future directions.Biomed Eng Online. 2023 Dec 16;22(1):126. doi: 10.1186/s12938-023-01187-8. Biomed Eng Online. 2023. PMID: 38102597 Free PMC article. Review.
-
Recent advances in the application of artificial intelligence in age-related macular degeneration.BMJ Open Ophthalmol. 2024 Nov 13;9(1):e001903. doi: 10.1136/bmjophth-2024-001903. BMJ Open Ophthalmol. 2024. PMID: 39537399 Free PMC article. Review.
References
-
- Teo Z.L., Tham Y.C., Yu M., et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–1591. - PubMed
-
- Beagley J., Guariguata L., Weil C., Motala A.A. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2015;103:150–160. - PubMed
-
- Klaver C.C., Wolfs R.C., Assink J.J., et al. Genetic risk of age-related maculopathy: population-based familial aggregation study. Arch. Ophthalmol. 1998;116:1646–1651. - PubMed
-
- Rosenfeld P.J., Brown D.M., Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
-
- Tham Y.C., Li X., Wong T.Y., et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. - PubMed
LinkOut - more resources
Full Text Sources

