Safety of AFM11 in the treatment of patients with B-cell malignancies: findings from two phase 1 studies
- PMID: 36597128
- PMCID: PMC9808944
- DOI: 10.1186/s13063-022-06982-7
Safety of AFM11 in the treatment of patients with B-cell malignancies: findings from two phase 1 studies
Abstract
Background: The prognosis for patients with relapsed and/or refractory (R/R) non-Hodgkin's lymphoma (NHL) or acute lymphoblastic leukaemia (ALL) remains poor, with existing treatments having significant side effects. Developed for the treatment of these cancers, AFM11 is a tetravalent, bispecific humanised recombinant antibody construct (TandAb®) designed to bind to human CD19 and CD3 and lead to the activation of T cells inducing apoptosis and killing of malignant B cells.
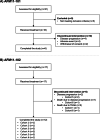
Methods: Two open-label, multicentre, dose-escalation phase 1 studies evaluated the safety, pharmacokinetics and activity of AFM11 in patients with R/R CD19-positive B cell NHL (AFM11-101) and in patients with CD19 + B-precursor Philadelphia-chromosome negative ALL (AFM11-102). Adverse events (AEs) were assessed and recorded; imaging (NHL) or bone marrow assessment (ALL) were used to evaluate response. Additional pharmacodynamic assays undertaken included cytokine release analysis and B-cell and T-cell depletion.
Results: In AFM11-101, 16 patients with R/R NHL received AFM11 in five different dose cohorts. Of which, 14 experienced drug-related treatment-emergent AEs (TEAEs) [including five serious AEs (SAEs)], five patients experienced dose-limiting toxicity (DLT) and ten patients discontinued the study. The high number of neurological events led to a decrease in infusion frequency during the study. No objective response to treatment was observed. In AFM11-102, 17 patients with R/R ALL received AFM11 in six different dose cohorts. Thirteen patients experienced drug-related TEAEs (including four SAEs), DLTs occurred in two patients and five patients discontinued the study. An objective response was recorded in three patients. The maximum tolerated dose could not be determined in either study due to early termination.
Conclusions: AFM11 treatment was associated with frequent neurological adverse reactions that were severe in some patients. In ALL, some signs of activity, albeit short-lived, were observed whereas no activity was observed in patients with NHL; therefore, further clinical development was terminated.
Trial registration: NCT02106091 . Safety Study to Assess AFM11 in Patients With Relapsed and/or Refractory CD19 Positive B-cell NHL. Registered April 2014. NCT02848911 . Safety Study to Assess AFM11 in Patients With Relapsed or Refractory Adult B-precursor ALL. Registered July 2016.
Keywords: AFM11; Acute lymphoblastic leukaemia; Neurotoxicity; Non-Hodgkin lymphoma; T-cell engager.
© 2023. The Author(s).
Conflict of interest statement
SES, KP and UG are employees of Affimed GmbH. AS and LA were employees of Affimed GmbH at the time of the study.
Figures
Similar articles
-
A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells.MAbs. 2015;7(3):584-604. doi: 10.1080/19420862.2015.1029216. MAbs. 2015. PMID: 25875246 Free PMC article.
-
Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients With Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study.J Clin Oncol. 2016 Apr 1;34(10):1104-11. doi: 10.1200/JCO.2014.59.1586. Epub 2016 Feb 16. J Clin Oncol. 2016. PMID: 26884582 Clinical Trial.
-
Functionally Defective T Cells After Chemotherapy of B-Cell Malignancies Can Be Activated by the Tetravalent Bispecific CD19/CD3 Antibody AFM11.J Immunother. 2019 Jun;42(5):180-188. doi: 10.1097/CJI.0000000000000267. J Immunother. 2019. PMID: 31090657
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy.Leuk Lymphoma. 2016 May;57(5):1021-32. doi: 10.3109/10428194.2016.1161185. Epub 2016 Apr 6. Leuk Lymphoma. 2016. PMID: 27050240 Review.
Cited by
-
T-Cell Engagers-The Structure and Functional Principle and Application in Hematological Malignancies.Cancers (Basel). 2024 Apr 20;16(8):1580. doi: 10.3390/cancers16081580. Cancers (Basel). 2024. PMID: 38672662 Free PMC article. Review.
-
Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies.J Hematol Oncol. 2023 Jul 27;16(1):83. doi: 10.1186/s13045-023-01482-w. J Hematol Oncol. 2023. PMID: 37501154 Free PMC article. Review.
-
Bispecific Antibodies in Hematological Malignancies: A Scoping Review.Cancers (Basel). 2023 Sep 14;15(18):4550. doi: 10.3390/cancers15184550. Cancers (Basel). 2023. PMID: 37760519 Free PMC article. Review.
-
Bispecific and multispecific antibodies in oncology: opportunities and challenges.Nat Rev Clin Oncol. 2024 Jul;21(7):539-560. doi: 10.1038/s41571-024-00905-y. Epub 2024 May 31. Nat Rev Clin Oncol. 2024. PMID: 38822215 Review.
-
New immune cell engagers for cancer immunotherapy.Nat Rev Immunol. 2024 Jul;24(7):471-486. doi: 10.1038/s41577-023-00982-7. Epub 2024 Jan 25. Nat Rev Immunol. 2024. PMID: 38273127 Review.
References
-
- Wild C, Weiderpass E, Stewart B, editors. World cancer report: cancer research for cancer prevention. Lyon: International Agency for Research on Cancer; 2020. http://publications.iarc.fr/586.
-
- National Institutes of Health. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Leukemia – Acute Lymphocytic Leukemia (ALL). https://seer.cancer.gov/statfacts/html/alyl.html.
-
- Gökbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101(12):1524–1533. doi: 10.3324/haematol.2016.144311. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

