Harnessing electromagnetic fields to assist bone tissue engineering
- PMID: 36631880
- PMCID: PMC9835389
- DOI: 10.1186/s13287-022-03217-z
Harnessing electromagnetic fields to assist bone tissue engineering
Abstract
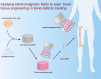
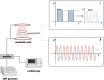
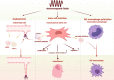
Bone tissue engineering (BTE) emerged as one of the exceptional means for bone defects owing to it providing mechanical supports to guide bone tissue regeneration. Great advances have been made to facilitate the success of BTE in regenerating bone within defects. The use of externally applied fields has been regarded as an alternative strategy for BTE. Electromagnetic fields (EMFs), known as a simple and non-invasive therapy, can remotely provide electric and magnetic stimulation to cells and biomaterials, thus applying EMFs to assist BTE would be a promising strategy for bone regeneration. When combined with BTE, EMFs improve cell adhesion to the material surface by promoting protein adsorption. Additionally, EMFs have positive effects on mesenchymal stem cells and show capabilities of pro-angiogenesis and macrophage polarization manipulation. These advantages of EMFs indicate that it is perfectly suitable for representing the adjuvant treatment of BTE. We also summarize studies concerning combinations of EMFs and diverse biomaterial types. The strategy of combining EMFs and BTE receives encouraging outcomes and holds a promising future for effectively treating bone defects.
Keywords: Bone regeneration; Bone tissue engineering; Electromagnetic fields; Osteogenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Sinusoidal electromagnetic fields accelerate bone regeneration by boosting the multifunctionality of bone marrow mesenchymal stem cells.Stem Cell Res Ther. 2021 Apr 13;12(1):234. doi: 10.1186/s13287-021-02302-z. Stem Cell Res Ther. 2021. PMID: 33849651 Free PMC article.
-
A comprehensive overview on utilizing electromagnetic fields in bone regenerative medicine.Electromagn Biol Med. 2019;38(1):1-20. doi: 10.1080/15368378.2019.1567527. Epub 2019 Jan 19. Electromagn Biol Med. 2019. PMID: 30661411 Review.
-
Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model.Sci Rep. 2018 Apr 20;8(1):6307. doi: 10.1038/s41598-018-24892-0. Sci Rep. 2018. PMID: 29679025 Free PMC article.
-
Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine.Int J Mol Sci. 2021 Sep 23;22(19):10233. doi: 10.3390/ijms221910233. Int J Mol Sci. 2021. PMID: 34638571 Free PMC article. Review.
-
Effects of electromagnetic fields on bone regeneration in experimental and clinical studies: a review of the literature.Chin Med J (Engl). 2012 Jan;125(2):367-72. Chin Med J (Engl). 2012. PMID: 22340573 Review.
Cited by
-
Harmonizing Magnetic Mitohormetic Regenerative Strategies: Developmental Implications of a Calcium-Mitochondrial Axis Invoked by Magnetic Field Exposure.Bioengineering (Basel). 2023 Oct 10;10(10):1176. doi: 10.3390/bioengineering10101176. Bioengineering (Basel). 2023. PMID: 37892906 Free PMC article. Review.
-
Bioelectricity in dental medicine: a narrative review.Biomed Eng Online. 2024 Jan 3;23(1):3. doi: 10.1186/s12938-023-01189-6. Biomed Eng Online. 2024. PMID: 38172866 Free PMC article. Review.
-
Microenvironment-_targeted strategy steers advanced bone regeneration.Mater Today Bio. 2023 Jul 21;22:100741. doi: 10.1016/j.mtbio.2023.100741. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37576867 Free PMC article. Review.
-
Magnetostrictive and Magnetoactive Effects in Piezoelectric Polymer Composites.Nanomaterials (Basel). 2023 Dec 21;14(1):31. doi: 10.3390/nano14010031. Nanomaterials (Basel). 2023. PMID: 38202485 Free PMC article.
-
Towards Stem Cell Therapy for Critical-Sized Segmental Bone Defects: Current Trends and Challenges on the Path to Clinical Translation.J Funct Biomater. 2024 May 27;15(6):145. doi: 10.3390/jfb15060145. J Funct Biomater. 2024. PMID: 38921519 Free PMC article. Review.
References
-
- McDermott AM, Herberg S, Mason DE, Collins JM, Pearson HB, Dawahare JH, Tang R, Patwa AN, Grinstaff MW, Kelly DJ, et al. Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci Transl Med. 2019;11(495):eaav7756. doi: 10.1126/scitranslmed.aav7756. - DOI - PMC - PubMed
-
- Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5(8):584–603. doi: 10.1038/s41578-020-0204-2. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

