Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light
- PMID: 36671781
- PMCID: PMC9855654
- DOI: 10.3390/biology12010089
Melatonin: Both a Messenger of Darkness and a Participant in the Cellular Actions of Non-Visible Solar Radiation of Near Infrared Light
Abstract
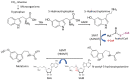
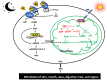
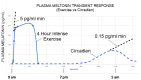
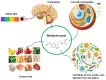
Throughout the history of melatonin research, almost exclusive focus has been on nocturnally-generated pineal melatonin production, which accounts for its circadian rhythm in the blood and cerebrospinal fluid; these light/dark melatonin cycles drive the daily and seasonal photoperiodic alterations in organismal physiology. Because pineal melatonin is produced and secreted primarily at night, it is referred to as the chemical expression of darkness. The importance of the other sources of melatonin has almost been ignored. Based on current evidence, there are at least four sources of melatonin in vertebrates that contribute to the whole-body melatonin pool. These include melatonin produced by (1) the pineal gland; (2) extrapineal cells, tissues, and organs; (3) the microbiota of the skin, mouth, nose, digestive tract, and vagina as well as (4) melatonin present in the diet. These multiple sources of melatonin exhibit differentially regulated mechanisms for its synthesis. Visible light striking the retina or an intense physical stimulus can suppress nocturnal pineal melatonin levels; in contrast, there are examples where extrapineal melatonin levels are increased during heavy exercise in daylight, which contains the whole range of NIR radiation. The cumulative impact of all cells producing augmented extrapineal melatonin is sufficient to elevate sweat concentrations, and potentially, if the exposure is sustained, to also increasing the circulating values. The transient increases in sweat and plasma melatonin support the premise that extrapineal melatonin has a production capacity that exceeds by far what can be produced by the pineal gland, and is used to maintain intercellular homeostasis and responds to rapid changes in ROS density. The potential regulatory mechanisms of near infrared light (NIR) on melatonin synthesis are discussed in detail herein. Combined with the discovery of high levels of melanopsin in most fat cells and their response to light further calls into question pineal centric theories. While the regulatory processes related to microbiota-derived melatonin are currently unknown, there does seem to be crosstalk between melatonin derived from the host and that originating from microbiota.
Keywords: cerebrospinal fluid; circadian rhythm; light; melatonin synthesis; microbiota; mitochondria; near-infrared radiation; pineal gland.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Intrinsically synthesized melatonin in mitochondria and factors controlling its production.Histol Histopathol. 2024 Jun 7:18776. doi: 10.14670/HH-18-776. Online ahead of print. Histol Histopathol. 2024. PMID: 38920277 Review.
-
CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal.Med Hypotheses. 2016 Jan;86:3-9. doi: 10.1016/j.mehy.2015.11.018. Epub 2015 Nov 26. Med Hypotheses. 2016. PMID: 26804589 Review.
-
Daily oscillation in melatonin synthesis in the Turkey pineal gland and retina: diurnal and circadian rhythms.Chronobiol Int. 2006;23(1-2):341-50. doi: 10.1080/07420520500482082. Chronobiol Int. 2006. PMID: 16687307
-
Diurnal and circadian rhythms in melatonin synthesis in the turkey pineal gland and retina.Gen Comp Endocrinol. 2006 Jan 15;145(2):162-8. doi: 10.1016/j.ygcen.2005.08.008. Epub 2005 Oct 13. Gen Comp Endocrinol. 2006. PMID: 16226264
-
Human pineal physiology and functional significance of melatonin.Front Neuroendocrinol. 2004 Sep-Dec;25(3-4):177-95. doi: 10.1016/j.yfrne.2004.08.001. Front Neuroendocrinol. 2004. PMID: 15589268 Review.
Cited by
-
Melatonin promotes hair regeneration by modulating the Wnt/β-catenin signalling pathway.Cell Prolif. 2024 Sep;57(9):e13656. doi: 10.1111/cpr.13656. Epub 2024 May 21. Cell Prolif. 2024. PMID: 38773710 Free PMC article.
-
The role of melatonergic system in intervertebral disc degeneration and its association with low back pain: a clinical study.PeerJ. 2024 Jul 9;12:e17464. doi: 10.7717/peerj.17464. eCollection 2024. PeerJ. 2024. PMID: 39006038 Free PMC article.
-
Light, Water, and Melatonin: The Synergistic Regulation of Phase Separation in Dementia.Int J Mol Sci. 2023 Mar 19;24(6):5835. doi: 10.3390/ijms24065835. Int J Mol Sci. 2023. PMID: 36982909 Free PMC article. Review.
-
The Importance of the Pineal Gland.Dtsch Arztebl Int. 2024 Aug 9;121(16):549. doi: 10.3238/arztebl.m2024.0028. Dtsch Arztebl Int. 2024. PMID: 39411910 Free PMC article. No abstract available.
-
Escherichia coli RimI Encodes Serotonin N-Acetyltransferase Activity and Its Overexpression Leads to Enhanced Growth and Melatonin Biosynthesis.Biomolecules. 2023 May 30;13(6):908. doi: 10.3390/biom13060908. Biomolecules. 2023. PMID: 37371488 Free PMC article.
References
-
- Lerner A.B., Case J.D., Takahashi Y., Lee Y., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587. doi: 10.1021/ja01543a060. - DOI
-
- Tan D.-X., Hardeland R., Manchester L.C., Paredes S.D., Korkmaz A., Sainz R.M., Mayo J.C., Fuentes-Broto L., Reiter R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010;85:607–623. doi: 10.1111/j.1469-185X.2009.00118.x. - DOI - PubMed
-
- Tan D.-X., Zheng X., Kong J., Manchester L., Hardeland R., Kim S., Xu X., Reiter R.J. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014;15:15858–15890. doi: 10.3390/ijms150915858. - DOI - PMC - PubMed
-
- Rosen J., Than N.N., Koch D., Poeggeler B., Laatsch H., Hardeland R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006;41:374–381. doi: 10.1111/j.1600-079X.2006.00379.x. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

