Studying Plant-Insect Interactions through the Analyses of the Diversity, Composition, and Functional Inference of Their Bacteriomes
- PMID: 36677331
- PMCID: PMC9863603
- DOI: 10.3390/microorganisms11010040
Studying Plant-Insect Interactions through the Analyses of the Diversity, Composition, and Functional Inference of Their Bacteriomes
Abstract
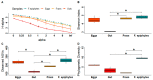
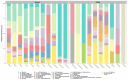
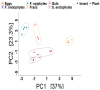
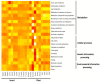
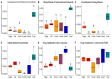
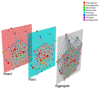
As with many other trophic interactions, the interchange of microorganisms between plants and their herbivorous insects is unavoidable. To test the hypothesis that the composition and diversity of the insect bacteriome are driven by the bacteriome of the plant, the bacteriomes of both the plant Datura inoxia and its specialist insect Lema daturaphila were characterised using 16S sRNA gene amplicon sequencing. Specifically, the bacteriomes associated with seeds, leaves, eggs, guts, and frass were described and compared. Then, the functions of the most abundant bacterial lineages found in the samples were inferred. Finally, the patterns of co-abundance among both bacteriomes were determined following a multilayer network approach. In accordance with our hypothesis, most genera were shared between plants and insects, but their abundances differed significantly within the samples collected. In the insect tissues, the most abundant genera were Pseudomonas (24.64%) in the eggs, Serratia (88.46%) in the gut, and Pseudomonas (36.27%) in the frass. In contrast, the most abundant ones in the plant were Serratia (40%) in seeds, Serratia (67%) in foliar endophytes, and Hymenobacter (12.85%) in foliar epiphytes. Indeed, PERMANOVA analysis showed that the composition of the bacteriomes was clustered by sample type (F = 9.36, p < 0.001). Functional inferences relevant to the interaction showed that in the plant samples, the category of Biosynthesis of secondary metabolites was significantly abundant (1.4%). In turn, the category of Xenobiotics degradation and metabolism was significantly present (2.5%) in the insect samples. Finally, the phyla Proteobacteria and Actinobacteriota showed a pattern of co-abundance in the insect but not in the plant, suggesting that the co-abundance and not the presence−absence patterns might be more important when studying ecological interactions.
Keywords: bacteriomes; co-abundance networks; diversity; foliar microbiota; functional inference; gut microbiota; plant–insect interaction.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Contrasting gut bacteriomes unveiled between wild Antheraea assamensis Helfer (Lepidoptera: Saturniidae) and domesticated Bombyx mori L. (Lepidoptera: Bombycidae) silkworms.Mol Biol Rep. 2024 May 22;51(1):666. doi: 10.1007/s11033-024-09629-9. Mol Biol Rep. 2024. PMID: 38777963
-
Bacterial Communities in Bacteriomes, Ovaries and Testes of three Geographical Populations of a Sap-Feeding Insect, Platypleura kaempferi (Hemiptera: Cicadidae).Curr Microbiol. 2021 May;78(5):1778-1791. doi: 10.1007/s00284-021-02435-7. Epub 2021 Mar 11. Curr Microbiol. 2021. PMID: 33704532
-
The oral bacteriomes of patients with allergic rhinitis and asthma differ from that of healthy controls.Front Microbiol. 2023 Jun 7;14:1197135. doi: 10.3389/fmicb.2023.1197135. eCollection 2023. Front Microbiol. 2023. PMID: 37440882 Free PMC article.
-
Plant-insect interactions under bacterial influence: ecological implications and underlying mechanisms.J Exp Bot. 2015 Feb;66(2):467-78. doi: 10.1093/jxb/eru435. Epub 2014 Nov 10. J Exp Bot. 2015. PMID: 25385767 Review.
-
The Gut Microbiota of the Insect Infraorder Pentatomomorpha (Hemiptera: Heteroptera) for the Light of Ecology and Evolution.Microorganisms. 2021 Feb 23;9(2):464. doi: 10.3390/microorganisms9020464. Microorganisms. 2021. PMID: 33672230 Free PMC article. Review.
Cited by
-
From phyllosphere to insect cuticles: silkworms gather antifungal bacteria from mulberry leaves to battle fungal parasite attacks.Microbiome. 2024 Feb 26;12(1):40. doi: 10.1186/s40168-024-01764-6. Microbiome. 2024. PMID: 38409012 Free PMC article.
References
-
- Mayoral-Peña Z., Álvarez-Martínez R., Fornoni J., Garrido E. The Extended Microbiota: How Microbes Shape Plant-Insect Interactions. Springer; Berlin/Heidelberg, Germany: 2020. pp. 135–146. - DOI
-
- Biere A., Tack A.J.M. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Func. Ecol. 2013;27:646–660. doi: 10.1111/1365-2435.12096. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

