Alleviation of imiquimod-induced psoriasis-like symptoms in Rorα-deficient mouse skin
- PMID: 36698281
- PMCID: PMC10230014
- DOI: 10.5483/BMBRep.2022-0169
Alleviation of imiquimod-induced psoriasis-like symptoms in Rorα-deficient mouse skin
Abstract
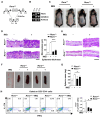
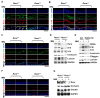
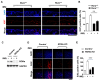
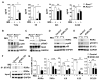
Retinoic acid receptor-related orphan receptor α (RORα) plays a vital role in various physiological processes, including metabolism, cancer, circadian rhythm, cerebellar development, and inflammation. Although RORα is expressed in the skin, its role in skin physiology remains poorly elucidated. Herein, Rorα was expressed in the basal and suprabasal layers of the epidermis; however, keratinocyte-specific Rorα deletion did not impact normal epidermal formation. Under pathophysiological conditions, Rorα-deficient mice exhibited alleviated psoriasis-like symptoms, including relatively intact epidermal stratification, reduced keratinocyte hyperproliferation, and low-level expression of inflammatory cytokines in keratinocytes. Unexpectedly, the splenic population of Th17 cells was significantly lower in keratinocytespecific RORα deficient mice than in the control. Additionally, Rorα-deficiency reduced imiquimod-induced activation of nuclear factor-κB and STAT3 in keratinocytes. Therefore, we expect that RORα inhibitors act on immune cells and keratinocytes to suppress the onset and progression of psoriasis.as an adjuvant for cancer immunotherapy. [BMB Reports 2023; 56(5): 296-301].
Conflict of interest statement
The authors have no conflicting interests.
Figures
Similar articles
-
Epidermal Loss of RORα Enhances Skin Inflammation in a MC903-Induced Mouse Model of Atopic Dermatitis.Int J Mol Sci. 2023 Jun 16;24(12):10241. doi: 10.3390/ijms241210241. Int J Mol Sci. 2023. PMID: 37373387 Free PMC article.
-
Specific Activation of CB2R Ameliorates Psoriasis-Like Skin Lesions by Inhibiting Inflammation and Oxidative Stress.Inflammation. 2023 Aug;46(4):1255-1271. doi: 10.1007/s10753-023-01805-6. Epub 2023 Mar 31. Inflammation. 2023. PMID: 37000322
-
PARP2 promotes inflammation in psoriasis by modulating estradiol biosynthesis in keratinocytes.J Mol Med (Berl). 2023 Aug;101(8):987-999. doi: 10.1007/s00109-023-02338-z. Epub 2023 Jun 23. J Mol Med (Berl). 2023. PMID: 37351597 Free PMC article.
-
Anoctamin1 Induces Hyperproliferation of HaCaT Keratinocytes and Triggers Imiquimod-Induced Psoriasis-Like Skin Injury in Mice.Int J Mol Sci. 2021 Jul 1;22(13):7145. doi: 10.3390/ijms22137145. Int J Mol Sci. 2021. PMID: 34281197 Free PMC article.
-
Hispidulin alleviates imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation.Int Immunopharmacol. 2020 Oct;87:106767. doi: 10.1016/j.intimp.2020.106767. Epub 2020 Jul 14. Int Immunopharmacol. 2020. PMID: 32679548
Cited by
-
Xiaoyin-anshen formula alleviates psoriasis complicated by sleep disturbances by regulating melatonin, antioxidant enzymes, and pro-inflammatory cytokines in mice.Front Pharmacol. 2024 Oct 1;15:1427985. doi: 10.3389/fphar.2024.1427985. eCollection 2024. Front Pharmacol. 2024. PMID: 39411067 Free PMC article.
-
Immune Portrayal of a New Therapy _targeting Microbiota in an Animal Model of Psoriasis.J Pers Med. 2023 Oct 30;13(11):1556. doi: 10.3390/jpm13111556. J Pers Med. 2023. PMID: 38003872 Free PMC article.
-
Epidermal Loss of RORα Enhances Skin Inflammation in a MC903-Induced Mouse Model of Atopic Dermatitis.Int J Mol Sci. 2023 Jun 16;24(12):10241. doi: 10.3390/ijms241210241. Int J Mol Sci. 2023. PMID: 37373387 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

