Cocoa Polyphenol Extract Inhibits Cellular Senescence via Modulation of SIRT1 and SIRT3 in Auditory Cells
- PMID: 36771251
- PMCID: PMC9921725
- DOI: 10.3390/nu15030544
Cocoa Polyphenol Extract Inhibits Cellular Senescence via Modulation of SIRT1 and SIRT3 in Auditory Cells
Abstract
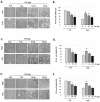
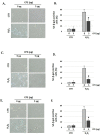
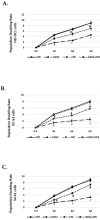
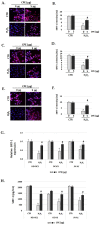
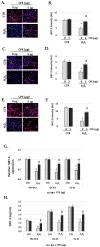
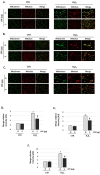
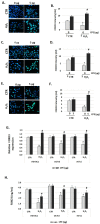
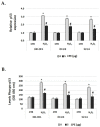
Cocoa, rich in polyphenols, has been reported to provide many health benefits due to its antioxidant properties. In this study, we investigated the effect of Cocoa polyphenols extract (CPE) against oxidative stress-induced cellular senescence using a hydrogen peroxide (H2O2)-induced cellular senescence model in three auditory cells lines derived from the auditory organ of a transgenic mouse: House Ear Institute-Organ of Corti 1 (HEI-OC1), Organ of Corti-3 (OC-k3), and Stria Vascularis (SV-k1) cells. Our results showed that CPE attenuated senescent phenotypes, including senescence-associated β-galactosidase expression, cell proliferation, alterations of morphology, oxidative DNA damage, mitochondrial dysfunction by inhibiting mitochondrial reactive oxygen species (mtROS) generation, and related molecules expressions such as forkhead box O3 (FOXO3) and p53. In addition, we determined that CPE induces expression of sirtuin 1 (SIRT1) and sirtuin 3 (SIRT3), and it has a protective role against cellular senescence by upregulation of SIRT1 and SIRT3. These data indicate that CPE protects against senescence through SIRT1, SIRT3, FOXO3, and p53 in auditory cells. In conclusion, these results suggest that Cocoa has therapeutic potential against age-related hearing loss (ARHL).
Keywords: Sirtuin 1; Sirtuin 3; Tumor suppressor protein p53; age-related hearing loss; cocoa; hydrogen peroxide (H2O2); mitochondrial reactive oxygen species; senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Preventive Effect of Cocoa Flavonoids via Suppression of Oxidative Stress-Induced Apoptosis in Auditory Senescent Cells.Antioxidants (Basel). 2022 Jul 26;11(8):1450. doi: 10.3390/antiox11081450. Antioxidants (Basel). 2022. PMID: 35892652 Free PMC article.
-
Luteolin inhibits H2O2-induced cellular senescence via modulation of SIRT1 and p53.Korean J Physiol Pharmacol. 2021 Jul 1;25(4):297-305. doi: 10.4196/kjpp.2021.25.4.297. Korean J Physiol Pharmacol. 2021. PMID: 34187948 Free PMC article.
-
Role of Oxidative Stress in the Senescence Pattern of Auditory Cells in Age-Related Hearing Loss.Antioxidants (Basel). 2021 Sep 21;10(9):1497. doi: 10.3390/antiox10091497. Antioxidants (Basel). 2021. PMID: 34573129 Free PMC article.
-
SIRT1 as a therapeutic _target in inflammaging of the pulmonary disease.Prev Med. 2012 May;54 Suppl(Suppl):S20-8. doi: 10.1016/j.ypmed.2011.11.014. Epub 2011 Dec 8. Prev Med. 2012. PMID: 22178470 Free PMC article. Review.
-
SIRT1 and p53, effect on cancer, senescence and beyond.Biochim Biophys Acta. 2010 Aug;1804(8):1684-9. doi: 10.1016/j.bbapap.2010.05.002. Epub 2010 May 13. Biochim Biophys Acta. 2010. PMID: 20471503 Free PMC article. Review.
Cited by
-
Plant-Derived Senotherapeutics for the Prevention and Treatment of Intervertebral Disc Degeneration and Aging.Metabolites. 2024 Feb 28;14(3):146. doi: 10.3390/metabo14030146. Metabolites. 2024. PMID: 38535306 Free PMC article. Review.
-
Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources.Biomolecules. 2023 Oct 31;13(11):1600. doi: 10.3390/biom13111600. Biomolecules. 2023. PMID: 38002283 Free PMC article. Review.
-
Microbiota-Derived Natural Products _targeting Cancer Stem Cells: Inside the Gut Pharma Factory.Int J Mol Sci. 2023 Mar 5;24(5):4997. doi: 10.3390/ijms24054997. Int J Mol Sci. 2023. PMID: 36902427 Free PMC article. Review.
-
Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences.Int J Mol Sci. 2024 Feb 23;25(5):2600. doi: 10.3390/ijms25052600. Int J Mol Sci. 2024. PMID: 38473850 Free PMC article. Review.
-
Roles of Sirtuins in Hearing Protection.Pharmaceuticals (Basel). 2024 Jul 28;17(8):998. doi: 10.3390/ph17080998. Pharmaceuticals (Basel). 2024. PMID: 39204103 Free PMC article. Review.
References
-
- Shimizu I., Yoshida Y., Katsuno T., Tateno K., Okada S., Moriya J., Yokoyama M., Nojima A., Ito T., Zechner R., et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15:51–64. doi: 10.1016/j.cmet.2011.12.006. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

