In vitro versus in situ biofilms for evaluating the antimicrobial effectiveness of herbal mouthrinses
- PMID: 36798085
- PMCID: PMC9927218
- DOI: 10.3389/fcimb.2023.1130255
In vitro versus in situ biofilms for evaluating the antimicrobial effectiveness of herbal mouthrinses
Abstract
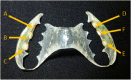
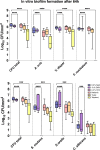
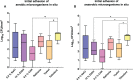
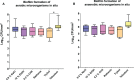
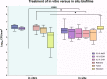
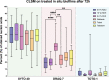
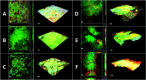
For centuries, diverse mouthrinses have been applied for medicinal purposes in the oral cavity. In view of the growing resistance of oral microorganisms against conventional antimicrobial agents e.g. chlorhexidine, the implementation of alternative treatments inspired by nature has lately gained increasing interest. The aim of the present study was to compare in vitro biofilm models with in situ biofilms in order to evaluate the antimicrobial potential of different natural mouthrinses. For the in vitro study a six-species supragingival biofilm model containing A. oris, V. dispar, C. albicans, F. nucleatum, S. mutans and S. oralis was used. Biofilms were grown anaerobically on hydroxyapatite discs and treated with natural mouthrinses Ratanhia, Trybol and Tebodont. 0.9% NaCl and 10% ethanol served as negative controls, while 0.2% CHX served as positive control. After 64h hours, biofilms were harvested and quantified by cultural analysis CFU. For the in situ study, individual test splints were manufactured for the participants. After 2h and 72h the biofilm-covered samples were removed and treated with the mouthrinses and controls mentioned above. The biofilms were quantified by CFU and stained for vitality under the confocal laser scanning microscope. In the in vitro study, 0.2% CHX yielded the highest antimicrobial effect. Among all mouthrinses, Tebodont (4.708 ± 1.294 log10 CFU, median 5.279, p<0.0001) compared with 0.9% NaCl showed the highest antimicrobial potential. After 72h there was no significant reduction in CFU after 0.2% CHX treatment. Only Trybol showed a statistically significant reduction of aerobic growth of microorganisms in situ (5.331 ± 0.7350 log10 CFU, median 5.579, p<0.0209). After treatment with the positive control 0.2% CHX, a significant percentage of non-vital bacteria (42.006 ± 12.173 log10 CFU, median 42.150) was detected. To sum up, a less pronounced effect of all mouthrinses was shown for the in situ biofilms compared to the in vitro biofilms.
Keywords: chlorhexidine (CHX); confocal laser scanning microscopy (CLSM); herbal mouthrinses; in situ; multispecies oral biofilm.
Copyright © 2023 Schönbächler, Thurnheer, Paqué, Attin and Karygianni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Characterization and application of a flow system for in vitro multispecies oral biofilm formation.J Periodontal Res. 2014 Jun;49(3):323-32. doi: 10.1111/jre.12110. Epub 2013 Jul 1. J Periodontal Res. 2014. PMID: 23815431
-
Comparative effect of chlorhexidine and some mouthrinses on bacterial biofilm formation on titanium surface.Curr Microbiol. 2011 Feb;62(2):445-51. doi: 10.1007/s00284-010-9727-x. Epub 2010 Aug 5. Curr Microbiol. 2011. PMID: 20686768
-
Fusobacterium Species and Subspecies Differentially Affect the Composition and Architecture of Supra- and Subgingival Biofilms Models.Front Microbiol. 2019 Jul 30;10:1716. doi: 10.3389/fmicb.2019.01716. eCollection 2019. Front Microbiol. 2019. PMID: 31417514 Free PMC article.
-
Low Concentrations of Chlorhexidine Inhibit the Formation and Structural Integrity of Enzyme-Treated Multispecies Oral Biofilms.Front Microbiol. 2021 Sep 28;12:741863. doi: 10.3389/fmicb.2021.741863. eCollection 2021. Front Microbiol. 2021. PMID: 34650542 Free PMC article.
-
Antibacterial activity of chlorhexidine after final irrigation with ethanol: CLSM and culture-based method analysis.Microsc Res Tech. 2015 Aug;78(8):682-7. doi: 10.1002/jemt.22525. Epub 2015 Jul 3. Microsc Res Tech. 2015. PMID: 26138134
Cited by
-
Formulation and assessment of biological properties of garcinia indica fruit extract mouthrinse as an adjunct to oral hygiene regimen: an in vitro analysis.J Appl Oral Sci. 2024 Jun 10;32:e20230291. doi: 10.1590/1678-7757-2023-0291. eCollection 2024. J Appl Oral Sci. 2024. PMID: 38865512 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

