Comparative Degradome Analysis of the Bovine Piroplasmid Pathogens Babesia bovis and Theileria annulata
- PMID: 36839509
- PMCID: PMC9965338
- DOI: 10.3390/pathogens12020237
Comparative Degradome Analysis of the Bovine Piroplasmid Pathogens Babesia bovis and Theileria annulata
Abstract
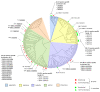
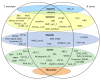
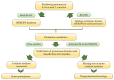
Babesia bovis and Theileria annulata are tick-borne hemoprotozoans that impact bovine health and are responsible for considerable fatalities in tropical and subtropical regions around the world. Both pathogens infect the same vertebrate host, are closely related, and contain similar-sized genomes; however, they differ in invertebrate host specificity, absence vs. presence of a schizont stage, erythrocyte invasion mechanism, and transovarial vs. transstadial transmission. Phylogenetic analysis and bidirectional best hit (BBH) identified a similar number of aspartic, metallo, and threonine proteinases and nonproteinase homologs. In contrast, a considerably increased number of S54 serine rhomboid proteinases and S9 nonproteinase homologs were identified in B. bovis, whereas C1A cysteine proteinases and A1 aspartic nonproteinase homologs were found to be expanded in T. annulata. Furthermore, a single proteinase of families S8 (subtilisin-like protein) and C12 (ubiquitin carboxyl-terminal hydrolase), as well as four nonproteinase homologs, one with dual domains M23-M23 and three with S9-S9, were exclusively present in B. bovis. Finally, a pronounced difference in species-specific ancillary domains was observed between both species. We hypothesize that the observed degradome differences represent functional correlates of the dissimilar life history features of B. bovis and T. annulata. The presented improved classification of piroplasmid proteinases will facilitate an informed choice for future in-depth functional studies.
Keywords: Babesia bovis; Theileria annulata; bovine babesiosis; comparative degradomics; degradome; peptidases; proteinase repertoire; tropical theileriosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cysteine Proteinase C1A Paralog Profiles Correspond with Phylogenetic Lineages of Pathogenic Piroplasmids.Vet Sci. 2018 Apr 17;5(2):41. doi: 10.3390/vetsci5020041. Vet Sci. 2018. PMID: 29673170 Free PMC article.
-
The repertoire of serine rhomboid proteases of piroplasmids of importance to animal and human health.Int J Parasitol. 2021 May;51(6):455-462. doi: 10.1016/j.ijpara.2020.10.010. Epub 2021 Feb 19. Int J Parasitol. 2021. PMID: 33610524
-
Establishment and application of a qPCR diagnostic method for Theileria annulata.Parasitol Res. 2022 Mar;121(3):973-980. doi: 10.1007/s00436-022-07434-6. Epub 2022 Jan 26. Parasitol Res. 2022. PMID: 35080659
-
The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights.Parasitol Res. 2022 May;121(5):1207-1245. doi: 10.1007/s00436-022-07424-8. Epub 2022 Jan 31. Parasitol Res. 2022. PMID: 35098377 Review.
-
Comparative Bioinformatics Analysis of Transcription Factor Genes Indicates Conservation of Key Regulatory Domains among Babesia bovis, Babesia microti, and Theileria equi.PLoS Negl Trop Dis. 2016 Nov 10;10(11):e0004983. doi: 10.1371/journal.pntd.0004983. eCollection 2016 Nov. PLoS Negl Trop Dis. 2016. PMID: 27832060 Free PMC article. Review.
Cited by
-
Investigation of Babesia spp. and Theileria spp. in ticks from Western China and identification of a novel genotype of Babesia caballi.BMC Vet Res. 2024 Jul 8;20(1):302. doi: 10.1186/s12917-024-04171-z. BMC Vet Res. 2024. PMID: 38978113 Free PMC article.
-
Cysteine Proteinase C1A Paralog Profiles Correspond with Phylogenetic Lineages of Pathogenic Piroplasmids.Vet Sci. 2018 Apr 17;5(2):41. doi: 10.3390/vetsci5020041. Vet Sci. 2018. PMID: 29673170 Free PMC article.
References
-
- Florin-Christensen M., Schnittger L., Bastos R.G., Rathinasamy V.A., Cooke B.M., Alzan H.F., Suarez C.E. Pursuing effective vaccines against cattle diseases caused by apicomplexan protozoa. CAB Rev. 2021;16:1–23. doi: 10.1079/PAVSNNR202116024. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

