Cyclooxygenase‑2 contributes to the hypoxia‑induced aggravation of the neuroinflammation response stimulated by lipopolysaccharide in microglia
- PMID: 36845947
- PMCID: PMC9947573
- DOI: 10.3892/etm.2023.11822
Cyclooxygenase‑2 contributes to the hypoxia‑induced aggravation of the neuroinflammation response stimulated by lipopolysaccharide in microglia
Abstract
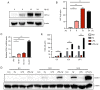
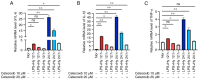
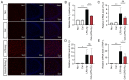
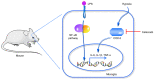
Hypoxia and neuroinflammation are key risk factors involved in various pathophysiological neural disorders. Hypoxia can aggravate neuroinflammation in vitro and in vivo; however, the underlying mechanisms remain unknown. In the present study, hypoxia [either 3 or 1% oxygen (O2)] increased lipopolysaccharide (LPS)-induced expression of the IL-6, IL-1β and TNF-α proinflammatory cytokines in BV2 cells. At the molecular level, both hypoxia and FG-4592, an hypoxia inducible factor 1 pathway activator, effectively induced cyclooxygenase-2 (COX-2) expression. The COX-2 inhibitor celecoxib significantly reduced the expression of cytokines induced by LPS under hypoxic conditions. Additionally, the administration of celecoxib inhibited the activation of microglia as well as cytokine expression in mice administered with hypoxia exposure and LPS injection. The present data demonstrated that COX-2 is involved in the hypoxia-induced aggravation of neuroinflammation stimulated by LPS.
Keywords: cyclooxygenase-2; hypoxia; lipopolysaccharide; microglia; neuroinflammation.
Copyright: © Yang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways.Phytomedicine. 2016 Dec 1;23(13):1629-1637. doi: 10.1016/j.phymed.2016.10.007. Epub 2016 Oct 14. Phytomedicine. 2016. PMID: 27823627
-
Hypoxia Potentiates LPS-Mediated Cytotoxicity of BV2 Microglial Cells In Vitro by Synergistic Effects on Glial Cytokine and Nitric Oxide System.Neuropediatrics. 2015 Oct;46(5):321-8. doi: 10.1055/s-0035-1562924. Epub 2015 Sep 10. Neuropediatrics. 2015. PMID: 26356486
-
Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation.Neurobiol Dis. 2020 Jul;140:104814. doi: 10.1016/j.nbd.2020.104814. Epub 2020 Feb 19. Neurobiol Dis. 2020. PMID: 32087283
-
The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation.J Neuroinflammation. 2015 Mar 19;12:54. doi: 10.1186/s12974-015-0268-x. J Neuroinflammation. 2015. PMID: 25889123 Free PMC article.
-
Chrysomycin A Attenuates Neuroinflammation by Down-Regulating NLRP3/Cleaved Caspase-1 Signaling Pathway in LPS-Stimulated Mice and BV2 Cells.Int J Mol Sci. 2021 Jun 24;22(13):6799. doi: 10.3390/ijms22136799. Int J Mol Sci. 2021. PMID: 34202695 Free PMC article.
Cited by
-
Hypoxia-Inducible Factor and Oxidative Stress in Tendon Degeneration: A Molecular Perspective.Antioxidants (Basel). 2024 Jan 10;13(1):86. doi: 10.3390/antiox13010086. Antioxidants (Basel). 2024. PMID: 38247510 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
