gBLUP-GWAS identifies candidate genes, signaling pathways, and putative functional polymorphisms for age at puberty in gilts
- PMID: 36848325
- PMCID: PMC10016198
- DOI: 10.1093/jas/skad063
gBLUP-GWAS identifies candidate genes, signaling pathways, and putative functional polymorphisms for age at puberty in gilts
Abstract
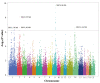
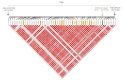
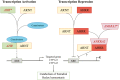
Successful development of replacement gilts determines their reproductive longevity and lifetime productivity. Selection for reproductive longevity is challenging due to low heritability and expression late in life. In pigs, age at puberty is the earliest known indicator for reproductive longevity and gilts that reach puberty earlier have a greater probability of producing more lifetime litters. Failure of gilts to reach puberty and display a pubertal estrus is a major reason for early removal of replacement gilts. To identify genomic sources of variation in age at puberty for improving genetic selection for early age at puberty and related traits, gilts (n = 4,986) from a multigeneration population representing commercially available maternal genetic lines were used for a genomic best linear unbiased prediction-based genome-wide association. Twenty-one genome-wide significant single nucleotide polymorphisms (SNP) located on Sus scrofa chromosomes (SSC) 1, 2, 9, and 14 were identified with additive effects ranging from -1.61 to 1.92 d (P < 0.0001 to 0.0671). Novel candidate genes and signaling pathways were identified for age at puberty. The locus on SSC9 (83.7 to 86.7 Mb) was characterized by long range linkage disequilibrium and harbors the AHR transcription factor gene. A second candidate gene on SSC2 (82.7 Mb), ANKRA2, is a corepressor for AHR, suggesting a possible involvement of AHR signaling in regulating pubertal onset in pigs. Putative functional SNP associated with age at puberty in the AHR and ANKRA2 genes were identified. Combined analysis of these SNP showed that an increase in the number of favorable alleles reduced pubertal age by 5.84 ± 1.65 d (P < 0.001). Candidate genes for age at puberty showed pleiotropic effects with other fertility functions such as gonadotropin secretion (FOXD1), follicular development (BMP4), pregnancy (LIF), and litter size (MEF2C). Several candidate genes and signaling pathways identified in this study play a physiological role in the hypothalamic-pituitary-gonadal axis and mechanisms permitting puberty onset. Variants located in or near these genes require further characterization to identify their impact on pubertal onset in gilts. Because age at puberty is an indicator of future reproductive success, these SNP are expected to improve genomic predictions for component traits of sow fertility and lifetime productivity expressed later in life.
Keywords: aryl hydrocarbon receptor; candidate gene; genome-wide association; genomic best linear unbiased prediction; puberty; swine.
Plain language summary
Selecting for replacement gilts is challenging because sow reproductive traits are lowly heritable and expressed late in life. Age at puberty is the earliest indicator of future reproductive success of gilts. Genetic selection for early onset of puberty could be feasible with the availability of molecular genetic predictors for age at puberty. To identify genomic sources associated with variation in age at puberty in gilts, a large-scale genome-wide association study was conducted at the U.S Meat Animal Research Center, Clay Center, Nebraska. Novel genomic associations for age at puberty were identified. Several candidate genes identified for age at puberty are involved in signaling pathways that regulate ovarian functions and pubertal onset. Potential causative genetic variants for age at puberty were identified within the candidate genes. These novel SNP are important new markers for use in genomic selection of replacement gilts with early puberty and provide critical new insight into biological mechanisms important for pubertal development in gilts.
Published by Oxford University Press on behalf of the American Society of Animal Science 2023.
Figures
Similar articles
-
GENOMICS SYMPOSIUM: Using genomic approaches to uncover sources of variation in age at puberty and reproductive longevity in sows.J Anim Sci. 2017 Sep;95(9):4196-4205. doi: 10.2527/jas2016.1334. J Anim Sci. 2017. PMID: 28992028
-
Genome-wide prediction of age at puberty and reproductive longevity in sows.Anim Genet. 2013 Aug;44(4):387-97. doi: 10.1111/age.12028. Epub 2013 Feb 26. Anim Genet. 2013. PMID: 23437861
-
Potential functional variants in AHR signaling pathways are associated with age at puberty in swine.Anim Genet. 2021 Jun;52(3):284-291. doi: 10.1111/age.13051. Epub 2021 Mar 5. Anim Genet. 2021. PMID: 33667011
-
Physiological and genomic insight into neuroendocrine regulation of puberty in gilts.Domest Anim Endocrinol. 2020 Oct;73:106446. doi: 10.1016/j.domaniend.2020.106446. Epub 2020 Feb 11. Domest Anim Endocrinol. 2020. PMID: 32199704 Review.
-
Functional genomics of reproduction in pigs: Are we there yet?Mol Reprod Dev. 2023 Jul;90(7):436-444. doi: 10.1002/mrd.23625. Epub 2022 Jun 15. Mol Reprod Dev. 2023. PMID: 35704517 Review.
Cited by
-
Relationships of genomic estimated breeding values for age at puberty, birth weight, and growth during development in normal cyclic and acyclic gilts.J Anim Sci. 2023 Jan 3;101:skad258. doi: 10.1093/jas/skad258. J Anim Sci. 2023. PMID: 37565572 Free PMC article.
References
-
- Barb, C. R., Kraeling R. R., and Rampacek G. B.. . 2001. Nutritional regulators of the hypothalamic-pituitary axis in pigs. In: Geisert, R. D., Niemann H., and Doberska C.. Reproduction Supplement 58, Control of Pig Reproduction VI. Cambridge, UK: Society for Reproduction and Fertility; p. 1–15. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

