A New Phenotype in Candida-Epithelial Cell Interaction Distinguishes Colonization- versus Vulvovaginal Candidiasis-Associated Strains
- PMID: 36856418
- PMCID: PMC10128025
- DOI: 10.1128/mbio.00107-23
A New Phenotype in Candida-Epithelial Cell Interaction Distinguishes Colonization- versus Vulvovaginal Candidiasis-Associated Strains
Abstract
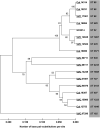
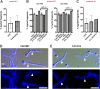
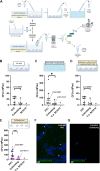
Vulvovaginal candidiasis (VVC) affects nearly 3/4 of women during their lifetime, and its symptoms seriously reduce quality of life. Although Candida albicans is a common commensal, it is unknown if VVC results from a switch from a commensal to pathogenic state, if only some strains can cause VVC, and/or if there is displacement of commensal strains with more pathogenic strains. We studied a set of VVC and colonizing C. albicans strains to identify consistent in vitro phenotypes associated with one group or the other. We find that the strains do not differ in overall genetic profile or behavior in culture media (i.e., multilocus sequence type [MLST] profile, rate of growth, and filamentation), but they show strikingly different behaviors during their interactions with vaginal epithelial cells. Epithelial infections with VVC-derived strains yielded stronger fungal proliferation and shedding of fungi and epithelial cells. Transcriptome sequencing (RNA-seq) analysis of representative epithelial cell infections with selected pathogenic or commensal isolates identified several differentially activated epithelial signaling pathways, including the integrin, ferroptosis, and type I interferon pathways; the latter has been implicated in damage protection. Strikingly, inhibition of type I interferon signaling selectively increases fungal shedding of strains in the colonizing cohort, suggesting that increased shedding correlates with lower interferon pathway activation. These data suggest that VVC strains may intrinsically have enhanced pathogenic potential via differential elicitation of epithelial responses, including the type I interferon pathway. Therefore, it may eventually be possible to evaluate pathogenic potential in vitro to refine VVC diagnosis. IMPORTANCE Despite a high incidence of VVC, we still have a poor understanding of this female-specific disease whose negative impact on women's quality of life has become a public health issue. It is not yet possible to determine by genotype or laboratory phenotype if a given Candida albicans strain is more or less likely to cause VVC. Here, we show that Candida strains causing VVC induce more fungal shedding from epithelial cells than strains from healthy women. This effect is also accompanied by increased epithelial cell detachment and differential activation of the type I interferon pathway. These distinguishing phenotypes suggest it may be possible to evaluate the VVC pathogenic potential of fungal isolates. This would permit more _targeted antifungal treatments to spare commensals and could allow for displacement of pathogenic strains with nonpathogenic colonizers. We expect these new assays to provide a more _targeted tool for identifying fungal virulence factors and epithelial responses that control fungal vaginitis.
Keywords: Candida albicans; epithelial cells; host-pathogen interactions; interferons; vulvovaginal candidiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro.Microbiol Spectr. 2022 Jun 29;10(3):e0269621. doi: 10.1128/spectrum.02696-21. Epub 2022 May 2. Microbiol Spectr. 2022. PMID: 35499353 Free PMC article.
-
In Vitro Antifungal Activity of Azoles and Other Antifungal Agents Against Pathogenic Yeasts from Vulvovaginal Candidiasis in China.Mycopathologia. 2023 Apr;188(1-2):99-109. doi: 10.1007/s11046-022-00687-w. Epub 2022 Nov 15. Mycopathologia. 2023. PMID: 36378354
-
Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome.mBio. 2015 Apr 21;6(2):e00182-15. doi: 10.1128/mBio.00182-15. mBio. 2015. PMID: 25900651 Free PMC article.
-
Molecular epidemiology, antifungal susceptibility, and ERG11 gene mutation of Candida species isolated from vulvovaginal candidiasis: Comparison between recurrent and non-recurrent infections.Microb Pathog. 2022 Sep;170:105696. doi: 10.1016/j.micpath.2022.105696. Epub 2022 Jul 31. Microb Pathog. 2022. PMID: 35921954 Review.
-
Virulence Factors of Candida spp. and Host Immune Response Important in the Pathogenesis of Vulvovaginal Candidiasis.Int J Mol Sci. 2022 May 24;23(11):5895. doi: 10.3390/ijms23115895. Int J Mol Sci. 2022. PMID: 35682581 Free PMC article. Review.
Cited by
-
Variations in candidalysin amino acid sequence influence toxicity and host responses.mBio. 2024 Aug 14;15(8):e0335123. doi: 10.1128/mbio.03351-23. Epub 2024 Jul 2. mBio. 2024. PMID: 38953356 Free PMC article.
-
Evolution and strain diversity advance exploration of Candida albicans biology.mSphere. 2024 Aug 28;9(8):e0064123. doi: 10.1128/msphere.00641-23. Epub 2024 Jul 16. mSphere. 2024. PMID: 39012122 Free PMC article. Review.
-
The Interplay between Candida albicans, Vaginal Mucosa, Host Immunity and Resident Microbiota in Health and Disease: An Overview and Future Perspectives.Microorganisms. 2023 May 5;11(5):1211. doi: 10.3390/microorganisms11051211. Microorganisms. 2023. PMID: 37317186 Free PMC article. Review.
-
Fungal burden, dimorphic transition and candidalysin: Role in Candida albicans-induced vaginal cell damage and mitochondrial activation in vitro.PLoS One. 2024 May 20;19(5):e0303449. doi: 10.1371/journal.pone.0303449. eCollection 2024. PLoS One. 2024. PMID: 38768097 Free PMC article.
-
Epithelial responses to fungal pathogens.Curr Opin Microbiol. 2024 Aug;80:102508. doi: 10.1016/j.mib.2024.102508. Epub 2024 Jul 10. Curr Opin Microbiol. 2024. PMID: 38986398 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

