The mast cell exosome-fibroblast connection: A novel pro-fibrotic pathway
- PMID: 36910476
- PMCID: PMC9995661
- DOI: 10.3389/fmed.2023.1139397
The mast cell exosome-fibroblast connection: A novel pro-fibrotic pathway
Abstract
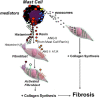
Introduction: In addition to the traditional activation of resident receptors by release of local mediators, new evidence favors the existence of exosomes in cell-to-cell communication that mediates delivery of specific cargo to modulate recipient cell function. We report that mast cell exosomes are an additional source of pro-fibrotic substances and constitute a unique pathway for the generation of excess collagen.
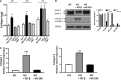
Methods: We use primary human lung fibroblasts (HLFs) to demonstrate the uptake of labeled exosomes isolated from the human mast cell line HMC-1 (MC-EXOs), previously shown to contain protein cargo in common with human mast cell exosomes.
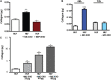
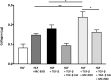
Results: The MC-EXO uptake by HLF is to the cytosol and increases both proline hydroxylation in HLF lysate and secreted collagen, within 24 h, which is sustained over 72 h, the same time required for transforming growth factor-β (TGF-β) to activate collagen synthesis in the HLFs. Unlike TGF-β, MC-EXO uptake does not induce fibrillar gene activation or invoke the Smad-nuclear transcription pathway. We show that MC-EXO uptake and TGF-β have an additive effect on collagen synthesis in HLF and postulate that MC-EXO uptake by HLFs is a contributing factor to excess collagen synthesis and represents a unique paradigm for understanding fibrosis.
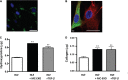
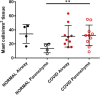
Discussion: It is known that, in the lungs, mast cells are more activated and increase in number with inflammation, injury and viral infection associated with fibrosis. With the reported increased incidence of post-COVID-pulmonary fibrosis (PCPF), data from patients with severe COVID-19 are presented that show an increase in the mast cell number in lung parenchyma, the site of PCPF. Our findings provide a rationale for _targeting multiple fibrogenic pathways in the management of lung fibrosis and the use of mast cell exosomes as a biomarker for the prognostic and diagnostic management of evolving fibrotic lung disease.
Keywords: exosomes; fibroblasts; fibrosis; lung; mast cells.
Copyright © 2023 Savage, Risquez, Gomi, Schreiner, Borczuk, Worgall and Silver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: role of transmembrane SCF and the PAR-2/PKC-α/Raf-1/p44/42 signaling pathway.Am J Pathol. 2013 Jun;182(6):2094-108. doi: 10.1016/j.ajpath.2013.02.013. Epub 2013 Apr 4. Am J Pathol. 2013. PMID: 23562441
-
CADM1 is a key receptor mediating human mast cell adhesion to human lung fibroblasts and airway smooth muscle cells.PLoS One. 2013 Apr 19;8(4):e61579. doi: 10.1371/journal.pone.0061579. Print 2013. PLoS One. 2013. PMID: 23620770 Free PMC article.
-
TGF-β pathway activation by idiopathic pulmonary fibrosis (IPF) fibroblast derived soluble factors is mediated by IL-6 trans-signaling.Respir Res. 2020 Feb 18;21(1):56. doi: 10.1186/s12931-020-1319-0. Respir Res. 2020. PMID: 32070329 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities.Mol Aspects Med. 2019 Feb;65:70-99. doi: 10.1016/j.mam.2018.07.001. Epub 2018 Aug 2. Mol Aspects Med. 2019. PMID: 30056242 Review.
Cited by
-
IL-10 Modulates the Expression and Activation of Pattern Recognition Receptors in Mast Cells.Int J Mol Sci. 2023 Jun 8;24(12):9875. doi: 10.3390/ijms24129875. Int J Mol Sci. 2023. PMID: 37373041 Free PMC article.
-
A survey of the currently known mast cell mediators with potential relevance for therapy of mast cell-induced symptoms.Naunyn Schmiedebergs Arch Pharmacol. 2023 Nov;396(11):2881-2891. doi: 10.1007/s00210-023-02545-y. Epub 2023 May 27. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37243761 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

