Mouse somatic cell nuclear transfer: What has changed and remained unchanged in 25 years
- PMID: 36928269
- PMCID: PMC10267587
- DOI: 10.1262/jrd.2022-105
Mouse somatic cell nuclear transfer: What has changed and remained unchanged in 25 years
Abstract
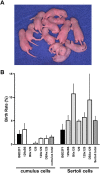
Somatic cell nuclear transfer (SCNT) is the only reproductive technology used to produce individuals from somatic cells by transferring them to enucleated oocytes. Although more than 25 years have passed since the first mammalian SCNT was reported in sheep, problems such as low birth rates and morphological abnormalities have persisted and limited its practical applications. The mouse is the ideal laboratory animal to unveil these questions due to its established reproductive technologies and extensive knowledge base of its genome and various strains. We investigated the causes of incomplete reprogramming after nuclear transfer of donor somatic cells and found that the loss of imprint in some placenta-specific imprinted genes could induce non-random SCNT abnormalities. By ameliorating aberrantly expressed imprinted genes, we succeeded in increasing the low birth rate and improving morphological abnormalities observed in SCNT fetuses. Furthermore, we sought appropriate mouse strains and cell types as nuclear donors to increase their developmental efficiencies and expand their applications in various fields. Peripheral blood cells are useful as ethical and economical cell species because they can be collected easily, even though SCNT embryos derived from hematopoietic cells show poor developmental abilities after reconstruction. Additionally, it is possible to obtain mice that are reactive to specific antigens of interest by using lymphocytes. Although there are still many limitations to the practical use of SCNT, its utilization is steadily expanding.
Keywords: Genome reprogramming; Imprinted gene; Mouse; Peripheral blood cell; Somatic cell nuclear transfer.
Conflict of interest statement
The authors declare no conflicts of interest associated with this manuscript.
Figures


Similar articles
-
25th ANNIVERSARY OF CLONING BY SOMATIC-CELL NUCLEAR TRANSFER: Epigenetic abnormalities associated with somatic cell nuclear transfer.Reproduction. 2021 Jun 11;162(1):F45-F58. doi: 10.1530/REP-21-0013. Reproduction. 2021. PMID: 33635828 Review.
-
Overexpression of PGC7 in donor cells maintains the DNA methylation status of imprinted genes in goat embryos derived from somatic cell nuclear transfer technology.Theriogenology. 2020 Jul 15;151:86-94. doi: 10.1016/j.theriogenology.2020.04.013. Epub 2020 Apr 15. Theriogenology. 2020. PMID: 32344274
-
Oct-4 activating compound 1 (OAC1) could improve the quality of somatic cell nuclear transfer embryos in the bovine.Theriogenology. 2023 Mar 1;198:75-86. doi: 10.1016/j.theriogenology.2022.11.002. Epub 2022 Nov 4. Theriogenology. 2023. PMID: 36565671
-
[Advances in epigenetic reprogramming of somatic cells nuclear transfer in mammals].Yi Chuan. 2019 Dec 20;41(12):1099-1109. doi: 10.16288/j.yczz.19-193. Yi Chuan. 2019. PMID: 31857281 Review. Chinese.
-
_targeting cellular memory to reprogram the epigenome, restore potential, and improve somatic cell nuclear transfer.Anim Reprod Sci. 2007 Mar;98(1-2):129-46. doi: 10.1016/j.anireprosci.2006.10.019. Epub 2006 Oct 21. Anim Reprod Sci. 2007. PMID: 17166676 Review.
Cited by
-
Essential roles of the nucleolus during early embryonic development: a regulatory hub for chromatin organization.Open Biol. 2024 May;14(5):230358. doi: 10.1098/rsob.230358. Epub 2024 May 1. Open Biol. 2024. PMID: 38689555 Free PMC article.
References
-
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 1958; 182: 64–65. - PubMed
-
- Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol 1962; 4: 256–273. - PubMed
-
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997; 385: 810–813. - PubMed
-
- Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394: 369–374. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

