Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies
- PMID: 36982424
- PMCID: PMC10048970
- DOI: 10.3390/ijms24065352
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing Antibodies
Abstract
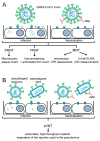
More than three years ago, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the unforeseen COVID-19 pandemic with millions of deaths. In the meantime, SARS-CoV-2 has become endemic and is now part of the repertoire of viruses causing seasonal severe respiratory infections. Due to several factors, among them the development of SARS-CoV-2 immunity through natural infection, vaccination and the current dominance of seemingly less pathogenic strains belonging to the omicron lineage, the COVID-19 situation has stabilized. However, several challenges remain and the possible new occurrence of highly pathogenic variants remains a threat. Here we review the development, features and importance of assays measuring SARS-CoV-2 neutralizing antibodies (NAbs). In particular we focus on in vitro infection assays and molecular interaction assays studying the binding of the receptor binding domain (RBD) with its cognate cellular receptor ACE2. These assays, but not the measurement of SARS-CoV-2-specific antibodies per se, can inform us of whether antibodies produced by convalescent or vaccinated subjects may protect against the infection and thus have the potential to predict the risk of becoming newly infected. This information is extremely important given the fact that a considerable number of subjects, in particular vulnerable persons, respond poorly to the vaccination with the production of neutralizing antibodies. Furthermore, these assays allow to determine and evaluate the virus-neutralizing capacity of antibodies induced by vaccines and administration of plasma-, immunoglobulin preparations, monoclonal antibodies, ACE2 variants or synthetic compounds to be used for therapy of COVID-19 and assist in the preclinical evaluation of vaccines. Both types of assays can be relatively quickly adapted to newly emerging virus variants to inform us about the magnitude of cross-neutralization, which may even allow us to estimate the risk of becoming infected by newly appearing virus variants. Given the paramount importance of the infection and interaction assays we discuss their specific features, possible advantages and disadvantages, technical aspects and not yet fully resolved issues, such as cut-off levels predicting the degree of in vivo protection.
Keywords: SARS-CoV-2; molecular assays; neutralizing antibodies; serological assays.
Conflict of interest statement
R.V. has received research grants from HVD Life-Sciences, Vienna, Austria, WORG Pharmaceuticals, Hangzhou, China and from Viravaxx AG, Vienna, Austria. He serves as consultant for Viravaxx AG. All other authors declare no conflict of interest.
Figures

Similar articles
-
Structural Basis of a Human Neutralizing Antibody Specific to the SARS-CoV-2 Spike Protein Receptor-Binding Domain.Microbiol Spectr. 2021 Oct 31;9(2):e0135221. doi: 10.1128/Spectrum.01352-21. Epub 2021 Oct 13. Microbiol Spectr. 2021. PMID: 34643438 Free PMC article.
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies _targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
An Assessment of Serological Assays for SARS-CoV-2 as Surrogates for Authentic Virus Neutralization.Microbiol Spectr. 2021 Oct 31;9(2):e0105921. doi: 10.1128/Spectrum.01059-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704832 Free PMC article.
-
Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection.J Adv Res. 2021 Jul;31:49-60. doi: 10.1016/j.jare.2020.12.013. Epub 2021 Jan 5. J Adv Res. 2021. PMID: 33520309 Free PMC article. Review.
-
Protective roles and protective mechanisms of neutralizing antibodies against SARS-CoV-2 infection and their potential clinical implications.Front Immunol. 2023 Jan 19;14:1055457. doi: 10.3389/fimmu.2023.1055457. eCollection 2023. Front Immunol. 2023. PMID: 36742320 Free PMC article. Review.
Cited by
-
Antigenic Cartography Indicates That the Omicron BA.1 and BA.4/BA.5 Variants Remain Antigenically Distant to Ancestral SARS-CoV-2 after Sputnik V Vaccination Followed by Homologous (Sputnik V) or Heterologous (Comirnaty) Revaccination.Int J Mol Sci. 2023 Jun 22;24(13):10493. doi: 10.3390/ijms241310493. Int J Mol Sci. 2023. PMID: 37445671 Free PMC article.
-
Ursodeoxycholic acid may protect from severe acute respiratory syndrome coronavirus 2 Omicron variant by reducing angiotensin-converting enzyme 2.Pharmacol Res Perspect. 2024 Apr;12(2):e1194. doi: 10.1002/prp2.1194. Pharmacol Res Perspect. 2024. PMID: 38573021 Free PMC article.
-
Identification of a Synthetic Polyhydroxyphenolic Resveratrol Analogue, 3,3',4,4',5,5'-Hexahydroxy-trans-Stilbene with Anti-SARS-CoV-2 Activity.Molecules. 2023 Mar 13;28(6):2612. doi: 10.3390/molecules28062612. Molecules. 2023. PMID: 36985582 Free PMC article.
-
Dissection of Antibody Responses of Gam-COVID-Vac-Vaccinated Subjects Suggests Involvement of Epitopes Outside RBD in SARS-CoV-2 Neutralization.Int J Mol Sci. 2023 Mar 7;24(6):5104. doi: 10.3390/ijms24065104. Int J Mol Sci. 2023. PMID: 36982183 Free PMC article.
-
A Pseudovirus-Based Neutralization Assay for SARS-CoV-2 Variants: A Rapid, Cost-Effective, BSL-2-Based High-Throughput Assay Useful for Vaccine Immunogenicity Evaluation.Microorganisms. 2024 Feb 29;12(3):501. doi: 10.3390/microorganisms12030501. Microorganisms. 2024. PMID: 38543552 Free PMC article.
References
-
- Nevejan L., Ombelet S., Laenen L., Keyaerts E., Demuyser T., Seyler L., Soetens O., Van Nedervelde E., Naesens R., Geysels D., et al. Severity of COVID-19 among Hospitalized Patients: Omicron Remains a Severe Threat for Immunocompromised Hosts. Viruses. 2022;14:2736. doi: 10.3390/v14122736. - DOI - PMC - PubMed
-
- Zerbit J., Detroit M., Meyer A., Decroocq J., Deau-Fischer B., Deschamps P., Birsen R., Mondesir J., Franchi P., Miekoutima E., et al. Patients with Hematological Malignancies Treated with T-Cell or B-Cell Immunotherapy Remain at High Risk of Severe Forms of COVID-19 in the Omicron Era. Viruses. 2022;14:2377. doi: 10.3390/v14112377. - DOI - PMC - PubMed
-
- Kratzer B., Trapin D., Gattinger P., Oberhofer T., Sehgal A.N.A., Waidhofer-Söllner P., Rottal A., Körmöczi U., Grabmeier-Pfistershammer K., Kopetzky G.H., et al. Lack of Induction of RBD-Specific Neutralizing Antibodies despite Repeated Heterologous SARS-CoV-2 Vaccination Leading to Seroconversion and Establishment of T Cell-Specific Memory in a Patient in Remission of Multiple Myeloma. Vaccines. 2022;10:374. doi: 10.3390/vaccines10030374. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

