Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles
- PMID: 36982481
- PMCID: PMC10056168
- DOI: 10.3390/ijms24065406
Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles
Abstract
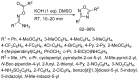
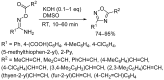
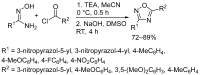
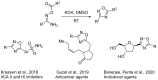
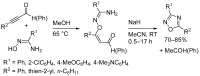
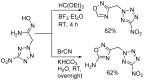
1,2,4-Oxadiazole is an essential motif in drug discovery represented in many experimental, investigational, and marketed drugs. This review covers synthetic methods that allow the conversion of different types of organic compounds into 1,2,4-oxadiazole at ambient temperature and the practical application of the latter approaches for the preparation of pharmaceutically important molecules. The discussed methods are divided into three groups. The first combines two-stage protocols requiring the preliminary preparation of O-acylamidoximes followed by cyclization under the action of organic bases. The advantages of this route are its swiftness, high efficiency of the cyclization process, and uncomplicated work-up. However, it requires the preparation and isolation of O-acylamidoximes as a separate preliminary step. The second route is a one-pot synthesis of 1,2,4-oxadiazoles directly from amidoximes and various carboxyl derivatives or aldehydes in aprotic bipolar solvents (primarily DMSO) in the presence of inorganic bases. This recently proposed pathway proved to be highly efficient in the field of medicinal chemistry. The third group of methods consists of diverse oxidative cyclizations, and these reactions have found modest application in drug design thus far. It is noteworthy that the reviewed methods allow for obtaining 1,2,4-oxadiazoles with thermosensitive functions and expand the prospects of using the oxadiazole core as an amide- or ester-like linker in the design of bioactive compounds.
Keywords: 1,2,4-oxadiazole; amidoximes; base catalyzed cyclization; bioactive compounds; bioisosteres; oxidative cyclization; room temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




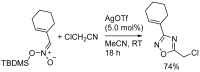
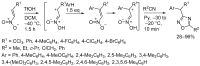
Similar articles
-
New Potential Biologically Active Compounds: Synthesis and Characterization of Urea and Thiourea Derivativpes Bearing 1,2,4-oxadiazole Ring.Curr Org Synth. 2020;17(7):525-534. doi: 10.2174/1570179417666200417112106. Curr Org Synth. 2020. PMID: 32303172
-
Substituted oxadiazoles: a patent review (2010 - 2012).Expert Opin Ther Pat. 2013 Sep;23(9):1209-32. doi: 10.1517/13543776.2013.797409. Epub 2013 May 10. Expert Opin Ther Pat. 2013. PMID: 23663160 Review.
-
A Highly Efficient Diversification of 2-Amino/Amido-1,3,4-oxadiazole and 1,3,4-Thiadiazole Derivatives via Reagent-Based Cyclization of Thiosemicarbazide Intermediate on Solid-Phase.ACS Comb Sci. 2015 Dec 14;17(12):732-41. doi: 10.1021/acscombsci.5b00140. Epub 2015 Nov 12. ACS Comb Sci. 2015. PMID: 26524617
-
A Review on Novel Synthesis Approaches and Biological Activities of 1,2,4- Oxadiazole and 1,3,4-Oxadiazole Tailored Compounds.Curr Org Synth. 2022 Aug 6;19(6):731-747. doi: 10.2174/1570179419666220216122238. Curr Org Synth. 2022. PMID: 35170412 Review.
-
Current Parallel Solid-Phase Synthesis of Drug-like Oxadiazole and Thiadiazole Derivatives for Combinatorial Chemistry.ACS Comb Sci. 2018 Jun 11;20(6):309-329. doi: 10.1021/acscombsci.8b00044. Epub 2018 May 7. ACS Comb Sci. 2018. PMID: 29714475 Review.
Cited by
-
Design, synthesis, and biological evaluation of multiple F-18 S1PR1 radiotracers in rodent and nonhuman primate.Org Biomol Chem. 2024 Jul 3;22(26):5428-5453. doi: 10.1039/d4ob00712c. Org Biomol Chem. 2024. PMID: 38884683
-
Hybrid 2D Supramolecular Organic Frameworks (SOFs) Assembled by the Cooperative Action of Hydrogen and Halogen Bonding and π⋯π Stacking Interactions.Int J Mol Sci. 2024 Feb 8;25(4):2062. doi: 10.3390/ijms25042062. Int J Mol Sci. 2024. PMID: 38396739 Free PMC article.
-
Bioactive Oxadiazoles 3.0.Int J Mol Sci. 2024 May 30;25(11):6027. doi: 10.3390/ijms25116027. Int J Mol Sci. 2024. PMID: 38892212 Free PMC article.
References
-
- Cohen J.A., Comi G., Selmaj K.W., Bar-Or A., Arnold D.L., Steinman L., Hartung H.-P., Montalban X., Kubala Havrdová E., Cree B.A.C., et al. Safety and Efficacy of Ozanimod versus Interferon Beta-1a in Relapsing Multiple Sclerosis (RADIANCE): A Multicentre, Randomised, 24-Month, Phase 3 Trial. Lancet Neurol. 2019;18:1021–1033. doi: 10.1016/S1474-4422(19)30238-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

