Factors associated with viral RNA shedding and evaluation of potential viral infectivity at returning to school in influenza outpatients after treatment with baloxavir marboxil and neuraminidase inhibitors during 2013/2014-2019/2020 seasons in Japan: an observational study
- PMID: 36991360
- PMCID: PMC10054210
- DOI: 10.1186/s12879-023-08140-z
Factors associated with viral RNA shedding and evaluation of potential viral infectivity at returning to school in influenza outpatients after treatment with baloxavir marboxil and neuraminidase inhibitors during 2013/2014-2019/2020 seasons in Japan: an observational study
Abstract
Background: This study assessed the differences in daily virus reduction and the residual infectivity after the recommended home stay period in Japan in patients infected with influenza and treated with baloxavir (BA), laninamivir (LA), oseltamivir (OS), and zanamivir (ZA).
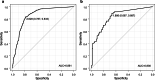
Methods: We conducted an observational study on children and adults at 13 outpatient clinics in 11 prefectures in Japan during seven influenza seasons from 2013/2014 to 2019/2020. Virus samples were collected twice from influenza rapid test-positive patients at the first and second visit 4-5 days after the start of treatment. The viral RNA shedding was quantified using quantitative RT-PCR. Neuraminidase (NA) and polymerase acidic (PA) variant viruses that reduce susceptibility to NA inhibitors and BA, respectively, were screened using RT-PCR and genetic sequencing. Daily estimated viral reduction was evaluated using univariate and multivariate analyses for the factors such as age, treatment, vaccination status, or the emergence of PA or NA variants. The potential infectivity of the viral RNA shedding at the second visit samples was determined using the Receiver Operator Curve based on the positivity of virus isolation.
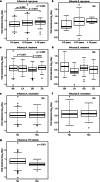
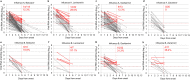
Results: Among 518 patients, 465 (80.0%) and 116 (20.0%) were infected with influenza A (189 with BA, 58 with LA, 181 with OS, 37 with ZA) and influenza B (39 with BA, 10 with LA, 52 with OS, 15 with ZA). The emergence of 21 PA variants in influenza A was detected after BA treatment, but NA variants were not detected after NAIs treatment. Multiple linear regression analysis showed that the daily viral RNA shedding reduction in patients was slower in the two NAIs (OS and LA) than in BA, influenza B infection, aged 0-5 years, or the emergence of PA variants. The residual viral RNA shedding potentially infectious was detected in approximately 10-30% of the patients aged 6-18 years after five days of onset.
Conclusions: Viral clearance differed by age, type of influenza, choice of treatment, and susceptibility to BA. Additionally, the recommended homestay period in Japan seemed insufficient, but reduced viral spread to some extent since most school-age patients became non-infectious after 5 days of onset.
Keywords: Baloxavir marboxil; Daily viral reduction; Influenza virus; Neuraminidase inhibitor; Viral RNA shedding.
© 2023. The Author(s).
Conflict of interest statement
All other authors report no conflict of interest.
Figures
Similar articles
-
Duration of fever in children infected with influenza A(H1N1)pdm09, A(H3N2) or B virus and treated with baloxavir marboxil, oseltamivir, laninamivir, or zanamivir in Japan during the 2012-2013 and 2019-2020 influenza seasons.Antiviral Res. 2024 Aug;228:105938. doi: 10.1016/j.antiviral.2024.105938. Epub 2024 Jun 17. Antiviral Res. 2024. PMID: 38897317
-
Virological and clinical outcomes in outpatients treated with baloxavir or oseltamivir: A Japanese multicenter study in the 2019-2020 influenza season.Antiviral Res. 2021 Aug;192:105092. doi: 10.1016/j.antiviral.2021.105092. Epub 2021 May 27. Antiviral Res. 2021. PMID: 34052230
-
Virological and Clinical Outcomes of Influenza Outpatients Treated With Baloxavir, Oseltamivir, or Laninamivir in the 2023-2024 Season.Influenza Other Respir Viruses. 2024 Nov;18(11):e70042. doi: 10.1111/irv.70042. Influenza Other Respir Viruses. 2024. PMID: 39557155 Free PMC article.
-
Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018-2020.Antiviral Res. 2022 Apr;200:105281. doi: 10.1016/j.antiviral.2022.105281. Epub 2022 Mar 12. Antiviral Res. 2022. PMID: 35292289 Free PMC article. Review.
-
Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children.Cochrane Database Syst Rev. 2012 Jan 18;1:CD008965. doi: 10.1002/14651858.CD008965.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Apr 10;(4):CD008965. doi: 10.1002/14651858.CD008965.pub4 PMID: 22258996 Updated. Review.
Cited by
-
Whole-Genome Analysis of the Influenza A(H1N1)pdm09 Viruses Isolated from Influenza-like Illness Outpatients in Myanmar and Community-Acquired Oseltamivir-Resistant Strains Present from 2015 to 2019.Viruses. 2024 Aug 15;16(8):1300. doi: 10.3390/v16081300. Viruses. 2024. PMID: 39205274 Free PMC article.
-
Bioinformatics analysis identifies a key gene HLA_DPA1 in severe influenza-associated immune infiltration.BMC Genomics. 2024 Mar 7;25(1):257. doi: 10.1186/s12864-024-10184-7. BMC Genomics. 2024. PMID: 38454348 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 22J23183/Grants-in-Aid for Scientific Research (KAKENHI)
- GC03220213/Community Medical Research Grant by the Niigata City Medical Association
- grant number not available/Tsukada Medical Research Grant
- 15fm0108009h0001-21wm0125005h0002/Japan Initiative for Global Research Network on Infectious Diseases
- 21K10414/KAKENHI
LinkOut - more resources
Full Text Sources
Medical

