Analysis of intracellular tyrosine phosphorylation in circulating neutrophils as a rapid assay for the in vivo effect of oral tyrosine kinase inhibitors
- PMID: 37089957
- PMCID: PMC10117656
- DOI: 10.3389/fphar.2023.1056154
Analysis of intracellular tyrosine phosphorylation in circulating neutrophils as a rapid assay for the in vivo effect of oral tyrosine kinase inhibitors
Abstract
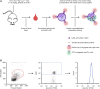
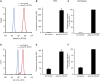
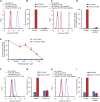
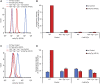
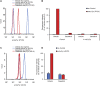
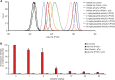
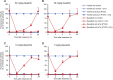
Tyrosine kinases are crucial signaling components of diverse biological processes and are major therapeutic _targets in various malignancies and immune-mediated disorders. A critical step of development of novel tyrosine kinase inhibitors is the transition from the confirmation of the in vitro effects of drug candidates to the analysis of their in vivo efficacy. To facilitate this transition, we have developed a rapid in vivo assay for the analysis of the effect of oral tyrosine kinase inhibitors on basal tyrosine phosphorylation of circulating mouse neutrophils. The assay uses a single drop of peripheral blood without sacrificing the mice. Flow cytometry using intracellular staining by fluorescently labeled anti-phosphotyrosine antibodies revealed robust basal tyrosine phosphorylation in resting circulating neutrophils. This signal was abrogated by the use of isotype control antibodies or by pre-saturation of the anti-phosphotyrosine antibodies with soluble phosphotyrosine amino acids or tyrosine-phosphorylated peptides. Basal tyrosine phosphorylation was dramatically reduced in neutrophils of triple knockout mice lacking the Src-family tyrosine kinases Hck, Fgr, and Lyn. Neutrophil tyrosine phosphorylation was also abrogated by oral administration of the Abl/Src-family inhibitor dasatinib, a clinically used anti-leukemic agent. Detailed dose-response and kinetic studies revealed half-maximal reduction of neutrophil tyrosine phosphorylation by 2.9 mg/kg dasatinib, with maximal reduction observed 2 h after inhibitor administration. Taken together, our assay allows highly efficient analysis of the in vivo effect of orally administered tyrosine kinase inhibitors, and may be used as a suitable alternative to other existing approaches.
Keywords: intracellular tyrosine phosphorylation; neutrophils; rapid in vivo assay; tyrosine kinase inhibitors (TKIs); tyrosine kinases.
Copyright © 2023 Futosi, Bajza, Deli, Erdélyi, Tusnády and Mócsai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Beta 2 integrin-dependent protein tyrosine phosphorylation and activation of the FGR protein tyrosine kinase in human neutrophils.J Cell Biol. 1994 Aug;126(4):1111-21. doi: 10.1083/jcb.126.4.1111. J Cell Biol. 1994. PMID: 7519620 Free PMC article.
-
Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases.J Biol Chem. 1996 Sep 20;271(38):23464-71. doi: 10.1074/jbc.271.38.23464. J Biol Chem. 1996. PMID: 8798554
-
CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A.J Immunol. 2004 Apr 15;172(8):5034-40. doi: 10.4049/jimmunol.172.8.5034. J Immunol. 2004. PMID: 15067085
-
Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases.J Immunol. 2000 Apr 15;164(8):4321-31. doi: 10.4049/jimmunol.164.8.4321. J Immunol. 2000. PMID: 10754332
-
Transmembrane signaling by the interleukin-2 receptor: progress and conundrums.Semin Immunol. 1993 Oct;5(5):345-64. doi: 10.1006/smim.1993.1041. Semin Immunol. 1993. PMID: 8260651 Review.
References
-
- Blaukat A. (2007). “Tyrosine kinases,” in xPharm: The comprehensive Pharmacology reference. Editors Enna S. J., Bylund D. B. (New York: Elsevier; ), 1–4.
-
- Brake K., Gumireddy A., Tiwari A., Chauhan H., Kumari D. (2017). In vivo studies for drug development via oral delivery: Challenges, animal models and techniques. Pharm. Anal. Acta 08. 10.4172/2153-2435.1000560 - DOI
-
- Chung T. D. Y., Terry D. B., Smith L. H., Markossian S., Grossman A., Brimacombe K., et al. (Editors) (2004). “ In vitro and in vivo assessment of ADME and PK properties during lead selection and lead optimization - guidelines, benchmarks and rules of thumb,” in Assay guidance manual (Bethesda (MD): Eli Lilly and Company and the National Center for Advancing Translational Sciences; ). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

