HSPB5 Inhibition by NCI-41356 Reduces Experimental Lung Fibrosis by Blocking TGF-β1 Signaling
- PMID: 37259327
- PMCID: PMC9960643
- DOI: 10.3390/ph16020177
HSPB5 Inhibition by NCI-41356 Reduces Experimental Lung Fibrosis by Blocking TGF-β1 Signaling
Abstract
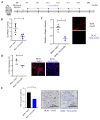
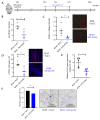
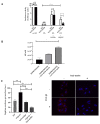
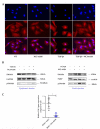
Idiopathic pulmonary fibrosis is a chronic, progressive and lethal disease of unknown etiology that ranks among the most frequent interstitial lung diseases. Idiopathic pulmonary fibrosis is characterized by dysregulated healing mechanisms that lead to the accumulation of large amounts of collagen in the lung tissue that disrupts the alveolar architecture. The two currently available treatments, nintedanib and pirfenidone, are only able to slow down the disease without being curative. We demonstrated in the past that HSPB5, a low molecular weight heat shock protein, was involved in the development of fibrosis and therefore was a potential therapeutic _target. Here, we have explored whether NCI-41356, a chemical inhibitor of HSPB5, can limit the development of pulmonary fibrosis. In vivo, we used a mouse model in which fibrosis was induced by intratracheal injection of bleomycin. Mice were treated with NaCl or NCI-41356 (six times intravenously or three times intratracheally). Fibrosis was evaluated by collagen quantification, immunofluorescence and TGF-β gene expression. In vitro, we studied the specific role of NCI-41356 on the chaperone function of HSPB5 and the inhibitory properties of NCI-41356 on HSPB5 interaction with its partner SMAD4 during fibrosis. TGF-β1 signaling was evaluated by immunofluorescence and Western Blot in epithelial cells treated with TGF-β1 with or without NCI-41356. In vivo, NCI-41356 reduced the accumulation of collagen, the expression of TGF-β1 and pro-fibrotic markers (PAI-1, α-SMA). In vitro, NCI-41356 decreased the interaction between HSPB5 and SMAD4 and thus modulated the SMAD4 canonical nuclear translocation involved in TGF-β1 signaling, which may explain NCI-41356 anti-fibrotic properties. In this study, we determined that inhibition of HSPB5 by NCI-41356 could limit pulmonary fibrosis in mice by limiting the synthesis of collagen and pro-fibrotic markers. At the molecular level, this outcome may be explained by the effect of NCI-41356 inhibiting HSPB5/SMAD4 interaction, thus modulating SMAD4 and TGF-β1 signaling. Further investigations are needed to determine whether these results can be transposed to humans.
Keywords: HSPB5; TGF-β; anti-fibrotic therapy; pulmonary fibrosis (PF).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
TRIM33 prevents pulmonary fibrosis by impairing TGF-β1 signalling.Eur Respir J. 2020 Jun 11;55(6):1901346. doi: 10.1183/13993003.01346-2019. Print 2020 Jun. Eur Respir J. 2020. PMID: 32184320
-
The small heat-shock protein αB-crystallin is essential for the nuclear localization of Smad4: impact on pulmonary fibrosis.J Pathol. 2014 Mar;232(4):458-72. doi: 10.1002/path.4314. J Pathol. 2014. PMID: 24307592
-
Pirfenidone and nintedanib attenuate pulmonary fibrosis in mice by inhibiting the expression of JAK2.J Thorac Dis. 2024 Feb 29;16(2):1128-1140. doi: 10.21037/jtd-23-1057. Epub 2024 Feb 26. J Thorac Dis. 2024. PMID: 38505034 Free PMC article.
-
Regulation of idiopathic pulmonary fibrosis: a cross-talk between TGF-β signaling and MicroRNAs.Front Med (Lausanne). 2024 Sep 25;11:1415278. doi: 10.3389/fmed.2024.1415278. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39386739 Free PMC article. Review.
-
Pulmonary fibrosis: pathogenesis and therapeutic strategies.MedComm (2020). 2024 Sep 23;5(10):e744. doi: 10.1002/mco2.744. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39314887 Free PMC article. Review.
Cited by
-
Advances and Challenges in _targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective.Pharmaceuticals (Basel). 2024 Apr 20;17(4):533. doi: 10.3390/ph17040533. Pharmaceuticals (Basel). 2024. PMID: 38675493 Free PMC article. Review.
-
Emerging delivery approaches for _targeted pulmonary fibrosis treatment.Adv Drug Deliv Rev. 2024 Jan;204:115147. doi: 10.1016/j.addr.2023.115147. Epub 2023 Dec 6. Adv Drug Deliv Rev. 2024. PMID: 38065244 Free PMC article. Review.
References
-
- Raghu G., Remy-Jardin M., Richeldi L., Thomson C.C., Inoue Y., Johkoh T., Kreuter M., Lynch D.A., Maher T.M., Martinez F.J., et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022;205:e18–e47. doi: 10.1164/rccm.202202-0399ST. - DOI - PMC - PubMed
-
- Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. - DOI - PubMed
-
- Bonniaud P., Kolb M., Galt T., Robertson J., Robbins C., Stampfli M., Lavery C., Margetts P.J., Roberts A.B., Gauldie J. Smad3 Null Mice Develop Airspace Enlargement and Are Resistant to TGF-Beta-Mediated Pulmonary Fibrosis. J. Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

