Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities
- PMID: 37373234
- PMCID: PMC10298922
- DOI: 10.3390/ijms241210084
Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities
Abstract
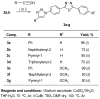
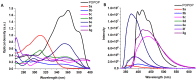
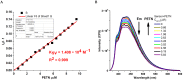
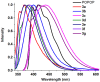
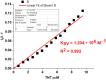
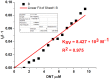
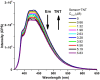
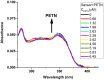
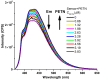
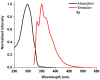
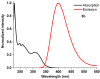
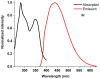
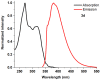
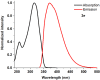
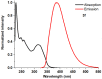
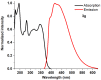
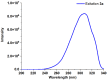
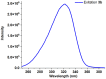
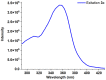
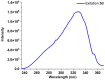
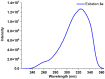
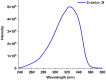
1,4-Bis(5-phenyl-2-oxazolyl)benzene (POPOP) is a common scintillation fluorescent laser dye. In this manuscript, the synthesis of 2-Ar-5-(4-(4-Ar'-1H-1,2,3-triazol-1-yl)phenyl)-1,3,4-oxadiazoles (Ar, Ar' = Ph, naphtalenyl-2, pyrenyl-1, triphenilenyl-2), as PAH-based aza-analogues of POPOP, by means of Cu-catalyzed click reaction between 2-(4-azidophenyl)-5-Ar-1,3,4-oxadiazole and terminal ethynyl-substituted PAHs is reported. An investigation of the photophysical properties of the obtained products was carried out, and their sensory response to nitroanalytes was evaluated. In the case of pyrenyl-1-substituted aza-POPOP, dramatic fluorescence quenching by nitroanalytes was observed.
Keywords: 1,4-bis(5-phenyl-2-oxazolyl)benzene analogues; aza-POPOPs; chemosensors; click reactions; fluorescence quenching; nitro-explosives; photophysical studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
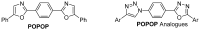
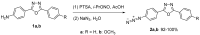

Similar articles
-
(1-(4-(5-Phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1H-1,2,3-triazol-4-yl)-methylenyls α,ω-Bisfunctionalized 3- and 4-PEG: Synthesis and Photophysical Studies.Molecules. 2023 Jul 6;28(13):5256. doi: 10.3390/molecules28135256. Molecules. 2023. PMID: 37446917 Free PMC article.
-
Synthesis, structural characterization, and photophysical, spectroelectrochemical, and anion-sensing studies of heteroleptic ruthenium(II) complexes derived from 4'-polyaromatic-substituted terpyridine derivatives and 2,6-bis(benzimidazol-2-yl)pyridine.Inorg Chem. 2013 Jun 17;52(12):6820-38. doi: 10.1021/ic3022326. Epub 2013 May 31. Inorg Chem. 2013. PMID: 23724852
-
Computer vision vs. spectrofluorometer-assisted detection of common nitro-explosive components with bola-type PAH-based chemosensors.RSC Adv. 2021 Jul 27;11(42):25850-25857. doi: 10.1039/d1ra03108b. eCollection 2021 Jul 27. RSC Adv. 2021. PMID: 35479431 Free PMC article.
-
Polyaromatic Hydrocarbons (PAHs): Structures, Synthesis and their Biological Profile.Curr Org Synth. 2020;17(8):625-640. doi: 10.2174/1570179417666200713182441. Curr Org Synth. 2020. PMID: 32660405 Review.
-
Aza-BODIPY-based Fluorescent and Colorimetric Sensors and Probes.Curr Org Synth. 2023;20(1):20-60. doi: 10.2174/1570179419666220216123033. Curr Org Synth. 2023. PMID: 35170414 Review.
Cited by
-
(1-(4-(5-Phenyl-1,3,4-oxadiazol-2-yl)phenyl)-1H-1,2,3-triazol-4-yl)-methylenyls α,ω-Bisfunctionalized 3- and 4-PEG: Synthesis and Photophysical Studies.Molecules. 2023 Jul 6;28(13):5256. doi: 10.3390/molecules28135256. Molecules. 2023. PMID: 37446917 Free PMC article.
References
-
- Adadurov A.F., Zhmurin P.N., Lebedev V.N., Titskaya V.D. Optimizing concentration of shifter additive for plastic scintillators of different size. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009;599:167–170. doi: 10.1016/j.nima.2008.11.014. - DOI
-
- Mardelli M., Olmsted J. Calorimetric determination of the 9,10-diphenyl-anthracene fluorescence quantum yield. J. Photochem. 1977;7:277–285. doi: 10.1016/0047-2670(77)85005-3. - DOI
-
- Shank C.V. Physics of dye lasers. Rev. Mod. Phys. 1975;47:649–657. doi: 10.1103/RevModPhys.47.649. - DOI
-
- Basov N.G., Logunov O.A., Startsev A.V., Stoilov Y.Y., Zuev V.S. Vapour phase dye lasers of the visible range. J. Mol. Struct. 1982;79:119–123. doi: 10.1016/0022-2860(82)85041-2. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

