Efficacy of intravenous immunoglobulins (IVIG) in COVID-19 patients: a systematic review and meta-analysis
- PMID: 37614613
- PMCID: PMC10443666
- DOI: 10.4103/1735-5362.378082
Efficacy of intravenous immunoglobulins (IVIG) in COVID-19 patients: a systematic review and meta-analysis
Abstract
Background and purpose: Though controversial, many clinical trials have been conducted to evaluate the efficacy of intravenous immunoglobulins (IVIG) in COVID-19 cases. Therefore, a systematic review and meta-analysis have been performed to evaluate the efficacy of IVIG in the treatment of COVID-19 patients.
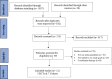
Experimental approach: A systematic search was performed in electronic databases and preprint servers up to November 20, 2021. Since substantial heterogeneity was expected, a random-effects model was applied to pool effect size from included studies to calculate the standardized mean differences (SMDs) for the continuous variables and relative risks (RRs) for the dichotomous variable with 95% confidence intervals (CIs).
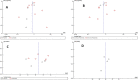
Findings/results: Five randomized clinical trials and seven cohort studies were analyzed among the 12 eligible studies with a total of 2,156 patients. The pooled RR of mortality was 0.77 (CI 0.59-1.01, P-value = 0.06), and of mechanical ventilation was 1.50 (CI 0.29-7.83; P-value = 0.63) in the IVIG group compared with the standard care group. The pooled SMD of hospital length of stay was 0.84 (CI -0.43-2.11; P-value = 0.20) and of ICU length of stay was -0.07 (CI -0.92-0.78; P-value = 0.86) in the IVIG group compared with the standard care group.
Conclusion and implications: This meta-analysis found that the IVIG therapy was not statistically different from the standard care group. Mortality, ICU admission, mechanical ventilation, length of hospital stay, and length of ICU stay were not significantly improved among IVIG recipients. However, statistical indifference is not equal to clinical indifference.
Keywords: Clinical efficacy; Intravenous immunoglobulin; Meta-analysis; Mortality rate; SARS-CoV-2 infection; Systematic review.
Copyright: © 2023 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Figures





Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The effect of intravenous immunoglobulins on the outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials.Expert Rev Anti Infect Ther. 2022 Oct;20(10):1333-1340. doi: 10.1080/14787210.2022.2098112. Epub 2022 Jul 7. Expert Rev Anti Infect Ther. 2022. PMID: 35786174
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2013 Jul 02;(7):CD000361. doi: 10.1002/14651858.CD000361.pub3 PMID: 14973955 Updated. Review.
-
Intravenous immunoglobulin for preventing infection in preterm and/or low-birth-weight infants.Cochrane Database Syst Rev. 2001;(2):CD000361. doi: 10.1002/14651858.CD000361. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2004;(1):CD000361. doi: 10.1002/14651858.CD000361.pub2 PMID: 11405962 Updated. Review.
-
Intravenous immunoglobulin for suspected or subsequently proven infection in neonates.Cochrane Database Syst Rev. 2004;(1):CD001239. doi: 10.1002/14651858.CD001239.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2010 Mar 17;(3):CD001239. doi: 10.1002/14651858.CD001239.pub3 PMID: 14973965 Updated. Review.
Cited by
-
Evaluation of the Efficacy and Safety of Intravenous Immunoglobulin (IVIG) in Moderate-to-Severe Hospitalized COVID-19 Patients: A Randomized, Open-Label Parallel-Group Study.Can J Infect Dis Med Microbiol. 2024 May 21;2024:7209380. doi: 10.1155/2024/7209380. eCollection 2024. Can J Infect Dis Med Microbiol. 2024. PMID: 38808260 Free PMC article.
References
-
- WHO. Coronavirus disease (COVID-19): weekly epidemiological update. World Health Organization. 2020. pp. 1–22. Available from: https://apps.who.int/iris/bitstream/handle/10665/334169/nCoV-weekly-sitr....
LinkOut - more resources
Full Text Sources
Miscellaneous
