Characterizing 3T3-L1 MBX Adipocyte Cell Differentiation Maintained with Fatty Acids as an In Vitro Model to Study the Effects of Obesity
- PMID: 37629569
- PMCID: PMC10455818
- DOI: 10.3390/life13081712
Characterizing 3T3-L1 MBX Adipocyte Cell Differentiation Maintained with Fatty Acids as an In Vitro Model to Study the Effects of Obesity
Abstract
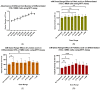
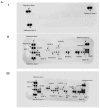
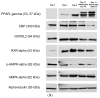
The increasing prevalence of obesity has prompted intensive research into understanding its role in pathogenesis and designing appropriate treatments. To determine the signals generated from the interaction of fat cells with a _target organ, a reliable white adipocyte model in vitro is needed. Differentiated fibroblasts are the most extensively studied using in vitro cell models of white adipocytes. However, it can be argued that differentiated fibroblasts minimally recapitulate the consequences of obesity. Here, we describe 3T3-L1 MBX cells as a culture model for studying obese adipocytes and their effects. Differentiation of 3T3-L1 MBX cells was at first optimized and then maintained in the presence of fatty acids cocktail combination to induce the obese condition. Lipid accumulation and adipokine secretion profiles were analyzed. Results showed that fatty acid-maintained, differentiated 3T3-L1 MBX cells had significantly greater accumulation of lipids and significant changes in the adipokine secretions in comparison to differentiated 3T3-L1 MBX cells maintained in medium without fatty acids. To elucidate the molecular changes associated with adipogenesis and lipid accumulation profile of 3T3-L1 MBX cells, we have also explored the expression of some of the regulatory proteins related to the development and maintenance of adipocytes from the preadipocyte lineage.
Keywords: 3T3-L1 MBX; adipocyte; adipocytokine; adipogenesis; in vitro fat cell model; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Cistanche tubulosa phenylethanoid glycosides suppressed adipogenesis in 3T3-L1 adipocytes and improved obesity and insulin resistance in high-fat diet induced obese mice.BMC Complement Med Ther. 2022 Oct 13;22(1):270. doi: 10.1186/s12906-022-03743-6. BMC Complement Med Ther. 2022. PMID: 36229811 Free PMC article.
-
Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells.Lipids Health Dis. 2017 Sep 25;16(1):181. doi: 10.1186/s12944-017-0574-7. Lipids Health Dis. 2017. PMID: 28946872 Free PMC article.
-
The role and possible mechanism of lncRNA U90926 in modulating 3T3-L1 preadipocyte differentiation.Int J Obes (Lond). 2017 Feb;41(2):299-308. doi: 10.1038/ijo.2016.189. Epub 2016 Oct 26. Int J Obes (Lond). 2017. PMID: 27780975 Free PMC article.
-
The role and possible mechanism of long noncoding RNA PVT1 in modulating 3T3-L1 preadipocyte proliferation and differentiation.IUBMB Life. 2020 Jul;72(7):1460-1467. doi: 10.1002/iub.2269. Epub 2020 Mar 9. IUBMB Life. 2020. PMID: 32150331
-
Acer tegmentosum Maxim Inhibits Adipogenesis in 3t3-l1 Adipocytes and Attenuates Lipid Accumulation in Obese Rats Fed a High-Fat Diet.Nutrients. 2020 Dec 7;12(12):3753. doi: 10.3390/nu12123753. Nutrients. 2020. PMID: 33297378 Free PMC article.
Cited by
-
The Effect of Adipocyte-Secreted Factors in Activating Focal Adhesion Kinase-Mediated Cell Signaling Pathway towards Metastasis in Breast Cancer Cells.Int J Mol Sci. 2023 Nov 22;24(23):16605. doi: 10.3390/ijms242316605. Int J Mol Sci. 2023. PMID: 38068928 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

