Plasma activated water as a pre-treatment strategy in the context of biofilm-infected chronic wounds
- PMID: 37771391
- PMCID: PMC10522953
- DOI: 10.1016/j.bioflm.2023.100154
Plasma activated water as a pre-treatment strategy in the context of biofilm-infected chronic wounds
Abstract
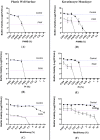
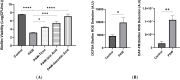
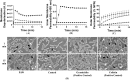
Healing and treatment of chronic wounds are often complicated due to biofilm formation by pathogens. Here, the efficacy of plasma activated water (PAW) as a pre-treatment strategy has been investigated prior to the application of topical antiseptics polyhexamethylene biguanide, povidone iodine, and MediHoney, which are routinely used to treat chronic wounds. The efficacy of this treatment strategy was determined against biofilms of Escherichia coli formed on a plastic substratum and on a human keratinocyte monolayer substratum used as an in vitro biofilm-skin epithelial cell model. PAW pre-treatment greatly increased the killing efficacy of all the three antiseptics to eradicate the E. coli biofilms formed on the plastic and keratinocyte substrates. However, the efficacy of the combined PAW-antiseptic treatment and single treatments using PAW or antiseptic alone was lower for biofilms formed in the in vitro biofilm-skin epithelial cell model compared to the plastic substratum. Scavenging assays demonstrated that reactive species present within the PAW were largely responsible for its anti-biofilm activity. PAW treatment resulted in significant intracellular reactive oxygen and nitrogen species accumulation within the E. coli biofilms, while also rapidly acting on the microbial membrane leading to outer membrane permeabilisation and depolarisation. Together, these factors contribute to significant cell death, potentiating the antibacterial effect of the assessed antiseptics.
Keywords: Antiseptics; Biofilm; Chronic wounds; Escherichia coli; In vitro; Plasma activated water.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
Patrick J. Cullen is the CEO of PlasmaLeap Technologies, the supplier of the plasma power source and BSD reactor utilised in this study.
Figures

Similar articles
-
Efficacy of antiseptics containing povidone-iodine, octenidine dihydrochloride and ethacridine lactate against biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus measured with the novel biofilm-oriented antiseptics test.Int Wound J. 2014 Dec;11(6):730-4. doi: 10.1111/iwj.12057. Epub 2013 Feb 28. Int Wound J. 2014. PMID: 23445335 Free PMC article.
-
[THE ROLE OF ANTISEPTICS AND STRATEGY OF BIOFILM REMOVAL IN CHRONIC WOUND].Acta Med Croatica. 2016 Mar;70(1):33-42. Acta Med Croatica. 2016. PMID: 27220188 Croatian.
-
Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-_targeting Antiseptics and Antibiotics.mBio. 2019 Jul 30;10(4):e01501-19. doi: 10.1128/mBio.01501-19. mBio. 2019. PMID: 31363032 Free PMC article.
-
Antiseptics for burns.Cochrane Database Syst Rev. 2017 Jul 12;7(7):CD011821. doi: 10.1002/14651858.CD011821.pub2. Cochrane Database Syst Rev. 2017. PMID: 28700086 Free PMC article. Review.
-
Antiseptics for treating infected wounds: Efficacy on biofilms and effect of pH.Crit Rev Microbiol. 2016;42(2):293-309. doi: 10.3109/1040841X.2014.940495. Epub 2014 Aug 27. Crit Rev Microbiol. 2016. PMID: 25159044 Review.
References
-
- O'Neill J. 2016. Tackling drug-resistant infections globally: final report and recommendations.
LinkOut - more resources
Full Text Sources

