Multinational patterns of second line antihyperglycaemic drug initiation across cardiovascular risk groups: federated pharmacoepidemiological evaluation in LEGEND-T2DM
- PMID: 37829182
- PMCID: PMC10565313
- DOI: 10.1136/bmjmed-2023-000651
Multinational patterns of second line antihyperglycaemic drug initiation across cardiovascular risk groups: federated pharmacoepidemiological evaluation in LEGEND-T2DM
Abstract
Objective: To assess the uptake of second line antihyperglycaemic drugs among patients with type 2 diabetes mellitus who are receiving metformin.
Design: Federated pharmacoepidemiological evaluation in LEGEND-T2DM.
Setting: 10 US and seven non-US electronic health record and administrative claims databases in the Observational Health Data Sciences and Informatics network in eight countries from 2011 to the end of 2021.
Participants: 4.8 million patients (≥18 years) across US and non-US based databases with type 2 diabetes mellitus who had received metformin monotherapy and had initiated second line treatments.
Exposure: The exposure used to evaluate each database was calendar year trends, with the years in the study that were specific to each cohort.
Main outcomes measures: The outcome was the incidence of second line antihyperglycaemic drug use (ie, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, dipeptidyl peptidase-4 inhibitors, and sulfonylureas) among individuals who were already receiving treatment with metformin. The relative drug class level uptake across cardiovascular risk groups was also evaluated.
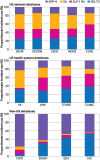
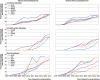
Results: 4.6 million patients were identified in US databases, 61 382 from Spain, 32 442 from Germany, 25 173 from the UK, 13 270 from France, 5580 from Scotland, 4614 from Hong Kong, and 2322 from Australia. During 2011-21, the combined proportional initiation of the cardioprotective antihyperglycaemic drugs (glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors) increased across all data sources, with the combined initiation of these drugs as second line drugs in 2021 ranging from 35.2% to 68.2% in the US databases, 15.4% in France, 34.7% in Spain, 50.1% in Germany, and 54.8% in Scotland. From 2016 to 2021, in some US and non-US databases, uptake of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors increased more significantly among populations with no cardiovascular disease compared with patients with established cardiovascular disease. No data source provided evidence of a greater increase in the uptake of these two drug classes in populations with cardiovascular disease compared with no cardiovascular disease.
Conclusions: Despite the increase in overall uptake of cardioprotective antihyperglycaemic drugs as second line treatments for type 2 diabetes mellitus, their uptake was lower in patients with cardiovascular disease than in people with no cardiovascular disease over the past decade. A strategy is needed to ensure that medication use is concordant with guideline recommendations to improve outcomes of patients with type 2 diabetes mellitus.
Keywords: Cardiology; Diabetes mellitus; Epidemiology; Pharmacology, clinical; Quality of health care.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This study is undertaken within Observational Health Data Sciences and Informatics, an open collaboration. RK received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (under award K23HL153775) and the Doris Duke Charitable Foundation (under award, 2022060). He is an Associate Editor of JAMA. He also receives research support, through Yale, from Bristol-Myers Squibb. He is a coinventor of US Provisional Patent Applications 63/177,117 and 63/346,610, unrelated to current work. He is also a founder of Evidence2Health, a precision health platform to improve evidence-based cardiovascular care. TD-S acknowledges receiving financial support from the Instituto de Salud Carlos III (ISCIII; Miguel Servet 2021: CP21/00023). KKCM reports grants from C W Maplethorpe Fellowship, grants from National Institute of Health Research, UK, grants from European Commission Framwork Horizon 2020, grants from Hong Kong Research Grant Council, grants from Innovation and Technology Commission of the Hong Kong Special Administration Region Government, and personal fees from IQVIA outside of the submitted work. DRM was supported by a Wellcome Trust Clinical Research Fellowship (214588/Z/18/Z). MJS is an employee and shareholder of Johnson & Johnson. HK received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Reality Labs, Tesseract/4Catalyst, F-Prime, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, and Martin/Baughman Law Firm. He is a co-founder of Refactor Health and HugoHealth, and is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare and Medicaid Services and through Yale University from Johnson & Johnson. MAS receives contracts and grants from the National Institutes of Health, the US Department of Veterans Affairs, the US Food and Drug Administration and Janssen Research and Development, the latter two of which are outside the scope of this work. Other authors declare no competing interests.
Figures


Similar articles
-
Large-scale evidence generation and evaluation across a network of databases for type 2 diabetes mellitus (LEGEND-T2DM): a protocol for a series of multinational, real-world comparative cardiovascular effectiveness and safety studies.BMJ Open. 2022 Jun 9;12(6):e057977. doi: 10.1136/bmjopen-2021-057977. BMJ Open. 2022. PMID: 35680274 Free PMC article.
-
Comparative Effectiveness of Second-line Antihyperglycemic Agents for Cardiovascular Outcomes: A Large-scale, Multinational, Federated Analysis of the LEGEND-T2DM Study.medRxiv [Preprint]. 2024 Feb 8:2024.02.05.24302354. doi: 10.1101/2024.02.05.24302354. medRxiv. 2024. Update in: J Am Coll Cardiol. 2024 Sep 3;84(10):904-917. doi: 10.1016/j.jacc.2024.05.069 PMID: 38370787 Free PMC article. Updated. Preprint.
-
Sex Differences in Cardiovascular Effectiveness of Newer Glucose-Lowering Drugs Added to Metformin in Type 2 Diabetes Mellitus.J Am Heart Assoc. 2020 Jan 7;9(1):e012940. doi: 10.1161/JAHA.119.012940. Epub 2020 Jan 4. J Am Heart Assoc. 2020. PMID: 31902326 Free PMC article.
-
Dipeptidyl-peptidase (DPP)-4 inhibitors and glucagon-like peptide (GLP)-1 analogues for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk for the development of type 2 diabetes mellitus.Cochrane Database Syst Rev. 2017 May 10;5(5):CD012204. doi: 10.1002/14651858.CD012204.pub2. Cochrane Database Syst Rev. 2017. PMID: 28489279 Free PMC article. Review.
-
Second-line Glucose-Lowering Therapy in Type 2 Diabetes Mellitus.Curr Diab Rep. 2019 Jul 8;19(8):54. doi: 10.1007/s11892-019-1171-0. Curr Diab Rep. 2019. PMID: 31286271 Review.
Cited by
-
Development and multinational validation of an algorithmic strategy for high Lp(a) screening.Nat Cardiovasc Res. 2024 May;3(5):558-566. doi: 10.1038/s44161-024-00469-1. Epub 2024 May 9. Nat Cardiovasc Res. 2024. PMID: 39195936
-
Artificial intelligence-enhanced patient evaluation: bridging art and science.Eur Heart J. 2024 Sep 14;45(35):3204-3218. doi: 10.1093/eurheartj/ehae415. Eur Heart J. 2024. PMID: 38976371 Review.
-
Machine learning in precision diabetes care and cardiovascular risk prediction.Cardiovasc Diabetol. 2023 Sep 25;22(1):259. doi: 10.1186/s12933-023-01985-3. Cardiovasc Diabetol. 2023. PMID: 37749579 Free PMC article. Review.
-
Cardiovascular Care Innovation through Data-Driven Discoveries in the Electronic Health Record.Am J Cardiol. 2023 Sep 15;203:136-148. doi: 10.1016/j.amjcard.2023.06.104. Epub 2023 Jul 25. Am J Cardiol. 2023. PMID: 37499593 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
