Eight Weeks of Daily Cannabidiol Supplementation Improves Sleep Quality and Immune Cell Cytotoxicity
- PMID: 37836465
- PMCID: PMC10574483
- DOI: 10.3390/nu15194173
Eight Weeks of Daily Cannabidiol Supplementation Improves Sleep Quality and Immune Cell Cytotoxicity
Abstract
Background: The endocannabinoid system is active in nervous and immune cells and involves the expression of two cannabinoid receptor genes (CB1 and CB2), along with endogenous endocannabinoid ligands, 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide (anandamide), and their synthetic enzymes. Cannabidiol (CBD) is a non-intoxicating exogenous cannabinoid agonist derived from plants that, at high doses, has received FDA approval as an anticonvulsant for epileptic seizures, and at low doses is marketed as a food-grade supplement for improved mental health, sleep quality, and immunological function. At present, the predominance of published CBD clinical research has focused on ameliorative or disease-specific intervention, with few trials investigating CBD effects in healthy populations.
Methods: This clinical study aimed to investigate the effects of 8 weeks of 50 mg oral CBD on mental health, sleep quantity and quality, and immune cell function in healthy, college-aged individuals. Twenty-eight participants (average age 25.9 ± 6.1 y) were randomized to receive either daily oral capsules of 50 mg of CBD (CB, n = 14) or a calorie-matched placebo (CN, n = 14). Participants completed pre- and post-intervention assessments, including anthropometric measurements, mental health surveys, sleep analysis, and immunological function assessments.
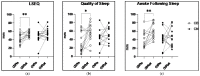
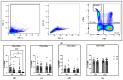
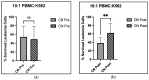
Results: After completing the 8-week intervention, there were no significant changes in body weight and BMI (CN: 1.09 ± 0.89%: CB: 1.41 ± 1.07%), or body fat percentage (CN: 9.01 ± 7.51%: CB: 8.57 ± 7.81%), respectively (values are % change pre to post, p > 0.05). There were also no significant differences between CB and CN groups with respect to mental health measures, sleep quantity, or circulating immunophenotype as a result of the intervention. However, the CB group experienced significant improvements in sleep quality measured objectively using a sleep questionnaire (p = 0.0023) and enhanced Natural Killer (NK) immune cell function assessed in situ (p = 0.0125).
Conclusions: Eight weeks of daily 50 mg CBD may improve sleep quality, and NK immunosurveillance in healthy, younger adults.
Keywords: cannabidiol; immunophenotype; mental health; natural killer cell; sleep; sleep quality.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The effects of a brand-specific, hemp-derived cannabidiol product on physiological, biochemical, and psychometric outcomes in healthy adults: a double-blind, randomized clinical trial.J Int Soc Sports Nutr. 2024 Dec;21(1):2370430. doi: 10.1080/15502783.2024.2370430. Epub 2024 Jun 21. J Int Soc Sports Nutr. 2024. PMID: 38904150 Free PMC article. Clinical Trial.
-
The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors.Pharmacol Res. 2017 May;119:358-370. doi: 10.1016/j.phrs.2017.02.022. Epub 2017 Feb 27. Pharmacol Res. 2017. PMID: 28249817
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The Endocannabinoid System Modulating Levels of Consciousness, Emotions and Likely Dream Contents.CNS Neurol Disord Drug _targets. 2017;16(4):370-379. doi: 10.2174/1871527316666170223161908. CNS Neurol Disord Drug _targets. 2017. PMID: 28240187 Review.
-
Potential antipsychotic properties of central cannabinoid (CB1) receptor antagonists.World J Biol Psychiatry. 2010 Mar;11(2 Pt 2):208-19. doi: 10.3109/15622970801908047. World J Biol Psychiatry. 2010. PMID: 20218784 Review.
Cited by
-
Cannabis-Based Phytocannabinoids: Overview, Mechanism of Action, Therapeutic Application, Production, and Affecting Environmental Factors.Int J Mol Sci. 2024 Oct 19;25(20):11258. doi: 10.3390/ijms252011258. Int J Mol Sci. 2024. PMID: 39457041 Free PMC article. Review.
-
Antimicrobial, Probiotic, and Immunomodulatory Potential of Cannabis sativa Extract and Delivery Systems.Antibiotics (Basel). 2024 Apr 17;13(4):369. doi: 10.3390/antibiotics13040369. Antibiotics (Basel). 2024. PMID: 38667045 Free PMC article.
References
-
- Terlizzi E.P., Schiller J.S. Mental Health Treatment among Adults Aged 18–44: United States, 2019–2021. NCHS; Hyattsville, MD, USA: 2022. pp. 1–8. NCHS Data Brief No. 444. - PubMed
-
- Consensus Conference Panel. Watson N.F., Badr M.S., Belenky G., Bliwise D.L., Buxton O.M., Buysse D., Dinges D.F., Gangwisch J., Grandner M.A., et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. J. Clin. Sleep Med. 2015;11:931–952. doi: 10.5665/sleep.4716. - DOI - PMC - PubMed
-
- Mechoulam R., Peters M., Murillo-Rodríguez E., Hanus L.O. Cannabidiol—Recent Advances. Chem. Biodivers. 2007;4:1678–1692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

