Insights into the function of ESCRT and its role in enveloped virus infection
- PMID: 37869652
- PMCID: PMC10587442
- DOI: 10.3389/fmicb.2023.1261651
Insights into the function of ESCRT and its role in enveloped virus infection
Abstract
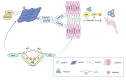
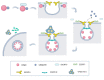
The endosomal sorting complex required for transport (ESCRT) is an essential molecular machinery in eukaryotic cells that facilitates the invagination of endosomal membranes, leading to the formation of multivesicular bodies (MVBs). It participates in various cellular processes, including lipid bilayer remodeling, cytoplasmic separation, autophagy, membrane fission and re-modeling, plasma membrane repair, as well as the invasion, budding, and release of certain enveloped viruses. The ESCRT complex consists of five complexes, ESCRT-0 to ESCRT-III and VPS4, along with several accessory proteins. ESCRT-0 to ESCRT-II form soluble complexes that shuttle between the cytoplasm and membranes, mainly responsible for recruiting and transporting membrane proteins and viral particles, as well as recruiting ESCRT-III for membrane neck scission. ESCRT-III, a soluble monomer, directly participates in vesicle scission and release, while VPS4 hydrolyzes ATP to provide energy for ESCRT-III complex disassembly, enabling recycling. Studies have confirmed the hijacking of ESCRT complexes by enveloped viruses to facilitate their entry, replication, and budding. Recent research has focused on the interaction between various components of the ESCRT complex and different viruses. In this review, we discuss how different viruses hijack specific ESCRT regulatory proteins to impact the viral life cycle, aiming to explore commonalities in the interaction between viruses and the ESCRT system.
Keywords: ESCRT; MVBS; enveloped virus; viral budding; viral entry; viral replication.
Copyright © 2023 Wang, Chen, Hu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The regulation of Endosomal Sorting Complex Required for Transport and accessory proteins in multivesicular body sorting and enveloped viral budding - An overview.Int J Biol Macromol. 2019 Apr 15;127:1-11. doi: 10.1016/j.ijbiomac.2019.01.015. Epub 2019 Jan 4. Int J Biol Macromol. 2019. PMID: 30615963 Review.
-
[Cellular ESCRT complex and its roles in enveloped viruses budding].Sheng Wu Gong Cheng Xue Bao. 2012 Sep;28(9):1031-7. Sheng Wu Gong Cheng Xue Bao. 2012. PMID: 23289305 Review. Chinese.
-
Distinct Roles of Cellular ESCRT-I and ESCRT-III Proteins in Efficient Entry and Egress of Budded Virions of Autographa californica Multiple Nucleopolyhedrovirus.J Virol. 2017 Dec 14;92(1):e01636-17. doi: 10.1128/JVI.01636-17. Print 2018 Jan 1. J Virol. 2017. PMID: 29046462 Free PMC article.
-
Dynamics of ESCRT proteins.Cell Mol Life Sci. 2012 Dec;69(24):4121-33. doi: 10.1007/s00018-012-1035-0. Epub 2012 Jun 6. Cell Mol Life Sci. 2012. PMID: 22669260 Free PMC article. Review.
-
Membrane scission by the ESCRT-III complex.Nature. 2009 Mar 12;458(7235):172-7. doi: 10.1038/nature07836. Epub 2009 Feb 22. Nature. 2009. PMID: 19234443 Free PMC article.
Cited by
-
Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design.Vaccines (Basel). 2024 Mar 7;12(3):280. doi: 10.3390/vaccines12030280. Vaccines (Basel). 2024. PMID: 38543914 Free PMC article. Review.
-
HRS Facilitates Newcastle Disease Virus Replication in Tumor Cells by Promoting Viral Budding.Int J Mol Sci. 2024 Sep 19;25(18):10060. doi: 10.3390/ijms251810060. Int J Mol Sci. 2024. PMID: 39337546 Free PMC article.
-
Research progress on the mechanism of exosome-mediated virus infection.Front Cell Infect Microbiol. 2024 Jun 26;14:1418168. doi: 10.3389/fcimb.2024.1418168. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38988816 Free PMC article. Review.
References
-
- Ahmed I., Akram Z., Iqbal H. M. N., Munn A. L. (2019). The regulation of endosomal sorting complex required for transport and accessory proteins in multivesicular body sorting and enveloped viral budding - an overview. Int. J. Biol. Macromol. 127, 1–11. doi: 10.1016/j.ijbiomac.2019.01.015, PMID: - DOI - PubMed
-
- Andrejeva J., Childs K. S., Young D. F., Carlos T. S., Stock N., Goodbourn S., et al. . (2004). The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 101, 17264–17269. doi: 10.1073/pnas.0407639101, PMID: - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

