Usp22 Deficiency Leads to Downregulation of PD-L1 and Pathological Activation of CD8+ T Cells and Causes Immunopathology in Response to Acute LCMV Infection
- PMID: 37896966
- PMCID: PMC10610587
- DOI: 10.3390/vaccines11101563
Usp22 Deficiency Leads to Downregulation of PD-L1 and Pathological Activation of CD8+ T Cells and Causes Immunopathology in Response to Acute LCMV Infection
Abstract
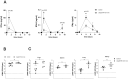
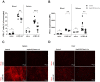
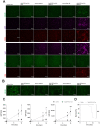
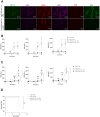
Ubiquitin-specific peptidase 22 (Usp22) cleaves ubiquitin moieties from numerous proteins, including histone H2B and transcription factors. Recently, it was reported that Usp22 acts as a negative regulator of interferon-dependent responses. In the current study, we investigated the role of Usp22 deficiency in acute viral infection with lymphocytic choriomeningitis virus (LCMV). We found that the lack of Usp22 on bone marrow-derived cells (Usp22fl/fl Vav1-Cre mice) reduced the induction of type I and II interferons. A limited type I interferon response did not influence virus replication. However, restricted expression of PD-L1 led to increased frequencies of functional virus-specific CD8+ T cells and rapid death of Usp22-deficient mice. CD8+ T cell depletion experiments revealed that accelerated CD8+ T cells were responsible for enhanced lethality in Usp22 deficient mice. In conclusion, we found that the lack of Usp22 generated a pathological CD8+ T cell response, which gave rise to severe disease in mice.
Keywords: LCMV; PD-L1 downregulation; activation of CD8+ T cells; liver failure; ubiquitin-specific peptidase 22 (Usp22).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection.J Virol. 2020 Aug 31;94(18):e00795-20. doi: 10.1128/JVI.00795-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641478 Free PMC article.
-
Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus.J Exp Med. 1998 Jun 1;187(11):1903-20. doi: 10.1084/jem.187.11.1903. J Exp Med. 1998. PMID: 9607930 Free PMC article.
-
IRF9 Prevents CD8+ T Cell Exhaustion in an Extrinsic Manner during Acute Lymphocytic Choriomeningitis Virus Infection.J Virol. 2017 Oct 27;91(22):e01219-17. doi: 10.1128/JVI.01219-17. Print 2017 Nov 15. J Virol. 2017. PMID: 28878077 Free PMC article.
-
CD8 T cell responses to lymphocytic choriomeningitis virus in early growth response gene 1-deficient mice.J Immunol. 2004 Sep 15;173(6):3855-62. doi: 10.4049/jimmunol.173.6.3855. J Immunol. 2004. PMID: 15356133
-
Immune Evasion and Drug Resistance Mediated by USP22 in Cancer: Novel _targets and Mechanisms.Front Immunol. 2022 Jul 20;13:918314. doi: 10.3389/fimmu.2022.918314. eCollection 2022. Front Immunol. 2022. PMID: 35935969 Free PMC article. Review.
Cited by
-
Programmed Death Ligand 1 Regulatory Crosstalk with Ubiquitination and Deubiquitination: Implications in Cancer Immunotherapy.Int J Mol Sci. 2024 Mar 3;25(5):2939. doi: 10.3390/ijms25052939. Int J Mol Sci. 2024. PMID: 38474186 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

