MEK/ERK and PI3K/AKT pathway inhibitors affect the transformation of myelodysplastic syndrome into acute myeloid leukemia via H3K27me3 methylases and de‑methylases
- PMID: 37921060
- PMCID: PMC10631768
- DOI: 10.3892/ijo.2023.5588
MEK/ERK and PI3K/AKT pathway inhibitors affect the transformation of myelodysplastic syndrome into acute myeloid leukemia via H3K27me3 methylases and de‑methylases
Abstract
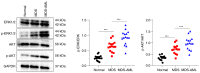
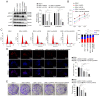
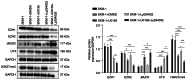
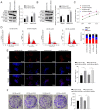
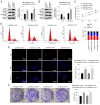
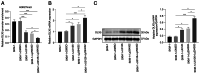
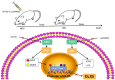
The transformation of myelodysplastic syndrome (MDS) into acute myeloid leukemia (AML) poses a significant clinical challenge. The trimethylation of H3 on lysine 27 (H3K27me3) methylase and de‑methylase pathway is involved in the regulation of MDS progression. The present study investigated the functional mechanisms of the MEK/ERK and PI3K/AKT pathways in the MDS‑to‑AML transformation. MDS‑AML mouse and SKM‑1 cell models were first established and this was followed by treatment with the MEK/ERK pathway inhibitor, U0126, the PI3K/AKT pathway inhibitor, Ly294002, or their combination. H3K27me3 methylase, enhancer of zeste homolog (EZH)1, EZH2, demethylase Jumonji domain‑containing protein‑3 (JMJD3) and ubiquitously transcribed tetratricopeptide repeat on chromosome X (UTX) and H3K27me3 protein levels were determined using western blot analysis. Cell viability, cycle distribution and proliferation were assessed using CCK‑8, flow cytometry, EdU and colony formation assays. The ERK and AKT phosphorylation levels in clinical samples and established models were determined, and SKM‑1 cell behaviors were assessed. The levels of H3K27me3 methylases and de‑methylases and distal‑less homeobox 5 (DLX5) were measured. The results revealed that the ERK and AKT phosphorylation levels were elevated in patients with MDS and MDS‑AML, and in mouse models. Treatment with U0126, a MEK/ERK pathway inhibitor, and Ly294002, a PI3K/AKT pathway inhibitor, effectively suppressed ERK and AKT phosphorylation in mice with MDS‑AML. It was observed that mice with MDS treated with U0126/Ly294002 exhibited reduced transformation to AML, delayed disease transformation and increased survival rates. Treatment of the SKM‑1 cells with U0126/Ly294002 led to a decrease in cell viability and proliferation, and to an increase in cell cycle arrest by suppressing ERK/PI3K phosphorylation. Moreover, treatment with U0126/Ly294002 downregulated EZH2/EZH1 expression, and upregulated JMJD3/UTX expression. The effects of U0126/Ly294002 were nullified when EZH2/EZH1 was overexpressed or when JMJD3/UTX was inhibited in the SKM‑1 cells. Treatment with U0126/Ly294002 also resulted in a decreased H3K27me3 protein level and H3K27me3 level in the DLX5 promoter region, leading to an increased DLX5 expression. Overall, the findings of the present study suggest that U0126/Ly294002 participates in MDS‑AML transformation by modulating the levels of H3K27me3 methylases and de‑methylases, and regulating DLX5 transcription and expression.
Keywords: H3K27me3; Ly294002; MEK/ERK; PI3K/AKT; SKM‑1 cells; U0126; acute myeloid leukemia; distal‑less homeobox 5; myelodysplastic syndrome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
[Cross-talk between PI3K/Akt and MEK/ERK pathways regulates human hepatocellular carcinoma cell cycle progression under endoplasmic reticulum stress].Zhonghua Gan Zang Bing Za Zhi. 2010 Dec;18(12):909-14. doi: 10.3760/cma.j.issn.1007-3418.2010.12.007. Zhonghua Gan Zang Bing Za Zhi. 2010. PMID: 21205476 Chinese.
-
SPAG6 silencing induces apoptosis in the myelodysplastic syndrome cell line SKM‑1 via the PTEN/PI3K/AKT signaling pathway in vitro and in vivo.Int J Oncol. 2018 Jul;53(1):297-306. doi: 10.3892/ijo.2018.4390. Epub 2018 May 2. Int J Oncol. 2018. PMID: 29749435
-
Effects of intravitreal insulin and insulin signaling cascade inhibitors on emmetropization in the chick.Mol Vis. 2012;18:2608-22. Epub 2012 Oct 20. Mol Vis. 2012. PMID: 23112573 Free PMC article.
-
Gene methylation in gastric cancer.Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review.
-
_targeting PI3K/AKT and MEK/ERK pathways for synergic effects on improving features of peripheral diabetic neuropathy.J Diabetes Investig. 2024 Nov;15(11):1537-1544. doi: 10.1111/jdi.14289. Epub 2024 Aug 20. J Diabetes Investig. 2024. PMID: 39162579 Free PMC article. Review.
Cited by
-
Inhibition of Enhancer of Zeste Homolog 2 Induces Blast Differentiation, Impairs Engraftment and Prolongs Survival in Murine Models of Acute Myeloid Leukemia.Cancers (Basel). 2024 Jan 29;16(3):569. doi: 10.3390/cancers16030569. Cancers (Basel). 2024. PMID: 38339323 Free PMC article.
-
Exploring and clinical validation of prognostic significance and therapeutic implications of copper homeostasis-related gene dysregulation in acute myeloid leukemia.Ann Hematol. 2024 Aug;103(8):2797-2826. doi: 10.1007/s00277-024-05841-6. Epub 2024 Jun 15. Ann Hematol. 2024. PMID: 38879648
-
The roles of phosphorylation of signaling proteins in the prognosis of acute myeloid leukemia.Pathol Oncol Res. 2024 Jul 5;30:1611747. doi: 10.3389/pore.2024.1611747. eCollection 2024. Pathol Oncol Res. 2024. PMID: 39035053 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
