Inhibition of UTX/KDM6A improves recovery of spinal cord injury by attenuating BSCB permeability and macrophage infiltration through the MLCK/p-MLC pathway
- PMID: 37951955
- PMCID: PMC10638785
- DOI: 10.1186/s12974-023-02936-1
Inhibition of UTX/KDM6A improves recovery of spinal cord injury by attenuating BSCB permeability and macrophage infiltration through the MLCK/p-MLC pathway
Abstract
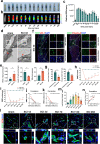
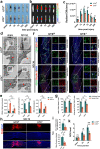
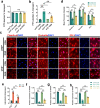
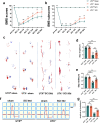
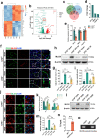
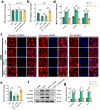
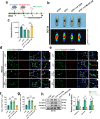
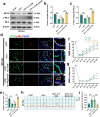
Spinal cord injury (SCI) can prompt an immediate disruption to the blood-spinal cord barrier (BSCB). Restoring the integrity of this barrier is vital for the recovery of neurological function post-SCI. The UTX protein, a histone demethylase, has been shown in previous research to promote vascular regeneration and neurological recovery in mice with SCI. However, it is unclear whether UTX knockout could facilitate the recovery of the BSCB by reducing its permeability. In this study, we systematically studied BSCB disruption and permeability at different time points after SCI and found that conditional UTX deletion in endothelial cells (ECs) can reduce BSCB permeability, decrease inflammatory cell infiltration and ROS production, and improve neurological function recovery after SCI. Subsequently, we used RNA sequencing and ChIP-qPCR to confirm that conditional UTX knockout in ECs can down-regulate expression of myosin light chain kinase (MLCK), which specifically mediates myosin light chain (MLC) phosphorylation and is involved in actin contraction, cell retraction, and tight junctions (TJs) protein integrity. Moreover, we found that MLCK overexpression can increase the ratio of p-MLC/MLC, further break TJs, and exacerbate BSCB deterioration. Overall, our findings indicate that UTX knockout could inhibit the MLCK/p-MLC pathway, resulting in decreased BSCB permeability, and ultimately promoting neurological recovery in mice. These results suggest that UTX is a promising new _target for treating SCI.
Keywords: Blood–spinal cord barrier; MYLK/MLCK; Spinal cord injury; UTX/KDM6A.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
Similar articles
-
Water treadmill training attenuates blood-spinal cord barrier disruption in rats by promoting angiogenesis and inhibiting matrix metalloproteinase-2/9 expression following spinal cord injury.Fluids Barriers CNS. 2020 Nov 25;17(1):70. doi: 10.1186/s12987-020-00232-1. Fluids Barriers CNS. 2020. PMID: 33292360 Free PMC article.
-
Perlecan Improves Blood Spinal Cord Barrier Repair Through the Integrin β1/ROCK/MLC Pathway After Spinal Cord Injury.Mol Neurobiol. 2023 Jan;60(1):51-67. doi: 10.1007/s12035-022-03041-9. Epub 2022 Oct 10. Mol Neurobiol. 2023. PMID: 36216996
-
Mertk Reduces Blood-Spinal Cord Barrier Permeability Through the Rhoa/Rock1/P-MLC Pathway After Spinal Cord Injury.Neurosci Bull. 2024 Sep;40(9):1230-1244. doi: 10.1007/s12264-024-01199-x. Epub 2024 Apr 9. Neurosci Bull. 2024. PMID: 38592581
-
Blood-Spinal Cord Barrier in Spinal Cord Injury: A Review.J Neurotrauma. 2021 May 1;38(9):1203-1224. doi: 10.1089/neu.2020.7413. Epub 2021 Feb 16. J Neurotrauma. 2021. PMID: 33292072 Review.
-
Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury.Mol Neurobiol. 2017 Jul;54(5):3578-3590. doi: 10.1007/s12035-016-9910-6. Epub 2016 May 18. Mol Neurobiol. 2017. PMID: 27194298 Review.
Cited by
-
MCT1-mediated endothelial cell lactate shuttle as a _target for promoting axon regeneration after spinal cord injury.Theranostics. 2024 Sep 3;14(14):5662-5681. doi: 10.7150/thno.96374. eCollection 2024. Theranostics. 2024. PMID: 39310103 Free PMC article.
-
Endothelial Foxo1 Phosphorylation Inhibition via Aptamer-Liposome Alleviates OPN-Induced Pathological Vascular Remodeling Following Spinal Cord Injury.Adv Sci (Weinh). 2024 Nov;11(43):e2406398. doi: 10.1002/advs.202406398. Epub 2024 Sep 28. Adv Sci (Weinh). 2024. PMID: 39340832 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

