High-Proportion Blue Light Irradiation at the End-of-Production Stage Promotes the Biosynthesis and Recycling of Ascorbate in Lettuce
- PMID: 38003716
- PMCID: PMC10671776
- DOI: 10.3390/ijms242216524
High-Proportion Blue Light Irradiation at the End-of-Production Stage Promotes the Biosynthesis and Recycling of Ascorbate in Lettuce
Abstract
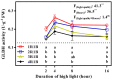
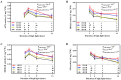
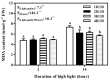
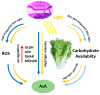
Ascorbate (AsA), an essential antioxidant for both plants and the human body, plays a vital role in maintaining proper functionality. Light plays an important role in metabolism of AsA in horticultural plants. Our previous research has revealed that subjecting lettuce to high light irradiation (HLI) (500 μmol·m-2·s-1) at the end-of-production (EOP) stage effectively enhances AsA levels, while the optimal light quality for AsA accumulation is still unknown. In this study, four combinations of red (R) and blue (B) light spectra with the ratio of 1:1 (1R1B), 2:1 (2R1B), 3:1 (3R1B), and 4:1 (4R1B) were applied to investigate the biosynthesis and recycling of AsA in lettuce. The results demonstrated that the AsA/total-AsA content in lettuce leaves was notably augmented upon exposure to 1R1B and 2R1B. Interestingly, AsA levels across all treatments increased rapidly at the early stage (2-8 h) of irradiation, while they increased slowly at the late stage (8-16 h). The activity of L-galactono-1,4-lactone dehydrogenase was augmented under 1R1B treatment, which is pivotal to AsA production. Additionally, the activities of enzymes key to AsA cycling were enhanced by 1R1B and 2R1B treatments, including ascorbate peroxidase, dehydroascorbate reductase, and monodehydroascorbate reductase. Notably, hydrogen peroxide and malondialdehyde accumulation increased dramatically following 16 h of 1R1B and 2R1B treatments. In addition, although soluble sugar and starch contents were enhanced by EOP-HLI, this effect was comparatively subdued under the 1R1B treatment. Overall, these results indicated that AsA accumulation was improved by irradiation with a blue light proportion of over 50% in lettuce, aligning with the heightened activities of key enzymes responsible for AsA synthesis, as well as the accrual of hydrogen peroxide. The effective strategy holds the potential to enhance the nutritional quality of lettuce while bolstering its antioxidant defenses.
Keywords: antioxidant; enzyme activity; light quality; plant factory; reactive oxygen species.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Dynamic Responses of Ascorbate Pool and Metabolism in Lettuce to Light Intensity at Night Time under Continuous Light Provided by Red and Blue LEDs.Plants (Basel). 2021 Jan 23;10(2):214. doi: 10.3390/plants10020214. Plants (Basel). 2021. PMID: 33498607 Free PMC article.
-
Effects of exogenous spermidine on antioxidants and glyoxalase system of lettuce seedlings under high temperature.Plant Signal Behav. 2020 Dec 1;15(12):1824697. doi: 10.1080/15592324.2020.1824697. Epub 2020 Sep 28. Plant Signal Behav. 2020. PMID: 32985921 Free PMC article.
-
Comparison of ascorbic acid biosynthesis in different tissues of three non-heading Chinese cabbage cultivars.Plant Physiol Biochem. 2013 Dec;73:229-36. doi: 10.1016/j.plaphy.2013.10.005. Epub 2013 Oct 10. Plant Physiol Biochem. 2013. PMID: 24157701
-
Regulation effects of hydrogen sulfide on ascorbate-glutathione cycle in naked oat leaves under saline-alkali stress.Ying Yong Sheng Tai Xue Bao. 2021 Nov 15;32(11):3988-3996. doi: 10.13287/j.1001-9332.202111.023. Ying Yong Sheng Tai Xue Bao. 2021. PMID: 34898115 English.
-
Ascorbic acid metabolism and functions: A comparison of plants and mammals.Free Radic Biol Med. 2018 Jul;122:116-129. doi: 10.1016/j.freeradbiomed.2018.03.033. Epub 2018 Mar 20. Free Radic Biol Med. 2018. PMID: 29567393 Free PMC article. Review.
Cited by
-
Continuous Blue Light Treatment Enhances the Nutritional Value of Hydroponically Grown Eruca vesicaria L. by Improving Ascorbic Acid Biosynthesis.Foods. 2024 Jul 5;13(13):2141. doi: 10.3390/foods13132141. Foods. 2024. PMID: 38998646 Free PMC article.
References
-
- Ntagkas N., Woltering E.J., Marcelis L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018;147:271–280. doi: 10.1016/j.envexpbot.2017.10.009. - DOI
-
- Laing W., Norling C., Di B., Wright M., Bulley S. Ascorbate concentration in Arabidopsis thaliana and expression of ascorbate related genes using RNAseq in response to light and the diurnal cycle. Cold Spring Harbor Labs J. 2017 doi: 10.1101/138008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

