A Bivalent Omicron-BA.4/BA.5-Adapted BNT162b2 Booster in ≥12-Year-Olds
- PMID: 38016021
- PMCID: PMC11093671
- DOI: 10.1093/cid/ciad718
A Bivalent Omicron-BA.4/BA.5-Adapted BNT162b2 Booster in ≥12-Year-Olds
Abstract
Background: Protection against contemporary severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants requires sequence-adapted vaccines.
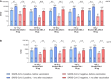
Methods: In this ongoing phase 2/3 trial, 12-17-year-olds (n = 108), 18-55-year-olds (n = 313), and >55-year-olds (n = 306) who previously received 3 original BNT162b2 30-µg doses, received a fourth dose (second booster) of 30-µg bivalent original/Omicron-BA.4/BA.5-adapted BNT162b2 (BNT162b2-Omi.BA.4/BA.5). For comparisons with original BNT162b2, participants were selected from another phase 3 trial. Immunologic superiority 1 month after vaccination, with respect to 50% neutralizing titers (lower bound [LB] of 2-sided 95% confidence interval [CI] for geometric mean ratio [GMR], >1), and noninferiority with respect to seroresponse rates (LB of 2-sided 95% CI for rate difference, greater than -5%), for Omicron BA.4/BA.5 were assessed in >55-year-olds versus original BNT162b2 as a second booster. Noninferiority with respect to neutralizing titer level (LB of 2-sided 95% CI for GMR, > 0.67) and seroresponse rate (LB of 2-sided 95% CI for rate difference, greater than -10%) of Omicron BA.4/BA.5 immune response for BNT162b2-Omi.BA.4/BA.5 in 18-55 versus >55-year-olds was assessed.
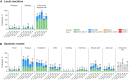
Results: One month after vaccination in >55-year-olds, the model-adjusted GMR of Omicron BA.4/BA.5 neutralizing titers for the BNT162b2-Omi.BA.4/BA.5 versus BNT162b2 groups (2.91 [95% CI, 2.45-3.44]) demonstrated the superiority of BNT162b2-Omi.BA.4/BA.5. Adjusted difference in the percentages of >55-year-olds with seroresponse (26.77% [95% CI, 19.59-33.95]) showed noninferiority of BNT162b2-Omi.BA.4/BA.5 to BNT162b2. Noninferiority of BNT162b2-Omi.BA.4/BA.5 in 18-55-year-olds compared with >55-year-olds was met for model-adjusted GMR and seroresponse. Geometric mean titers in 12-17-year-olds increased from baseline to 1 month after vaccination. The BNT162b2-Omi.BA.4/BA.5 safety profile was similar to the profiles for booster doses of bivalent Omicron BA.1-modified BNT162b2 and original BNT162b2 reported in previous studies.
Conclusions: Based on immunogenicity and safety data up to 1 month after vaccination in participants who previously received 3 original BNT162b2 doses, a BNT162b2-Omi.BA.4/BA.5 30-µg booster has a favorable benefit-risk profile.
Clinical trials registration: NCT05472038.
Keywords: BNT162b2 vaccine; Omicron variant; SARS-CoV-2; booster; immunogenicity.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . L. U. reports consulting fees made to institution from Pfizer Survey and a role as Memphis Medical Society President. S. P., X. X., D. Y. L., H. W., F. S. L., C. L., D. C., K. K., A. S. A., K. A. S., W. C. G., and N. K. are employees of Pfizer and may hold stock or stock options. H. R. reports consulting fees from Moderna for Vaccine advisory meeting. D. F. received funding from Pfizer for conducting research trials. F. J. M., Ö. T., and U. Ş. are employees of BioNTech and may hold stock or stock options. Ö. T. reports grants or contracts for joint development from Pfizer, is a coauthor on various issued or pending patents related to RNA technology and coronavirus disease 2019 (COVID-19) vaccines from BioNTech, and is a cofounder and chief executive officer of BioNTech. U. Ş. reports grants or contracts for joint development from Pfizer, is a coauthor on various issued or pending patents related to RNA technology and COVID-19 vaccines from BioNTech, and is a cofounder and chief executive officer of BioNTech. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Similar articles
-
Bivalent Omicron BA.4/BA.5 BNT162b2 Vaccine in 6-Month- to <12-Year-Olds.J Pediatric Infect Dis Soc. 2024 Aug 24;13(8):421-429. doi: 10.1093/jpids/piae062. J Pediatric Infect Dis Soc. 2024. PMID: 38860591 Free PMC article. Clinical Trial.
-
Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years.N Engl J Med. 2023 Jan 19;388(3):214-227. doi: 10.1056/NEJMoa2213082. N Engl J Med. 2023. PMID: 36652353 Free PMC article. Clinical Trial.
-
Booster vaccination using bivalent DS-5670a/b is safe and immunogenic against SARS-CoV-2 variants in children aged 5-11 years: a phase 2/3, randomized, active-controlled study.Front Immunol. 2024 Sep 2;15:1445459. doi: 10.3389/fimmu.2024.1445459. eCollection 2024. Front Immunol. 2024. PMID: 39286253 Free PMC article. Clinical Trial.
-
A review of the immunogenicity and safety of booster doses of omicron variant-containing mRNA-1273 COVID-19 vaccines in adults and children.Expert Rev Vaccines. 2024 Jan-Dec;23(1):862-878. doi: 10.1080/14760584.2024.2397026. Epub 2024 Sep 9. Expert Rev Vaccines. 2024. PMID: 39234779 Review.
-
A Brighton Collaboration standardized template with key considerations for a benefit-risk assessment for the Comirnaty COVID-19 mRNA vaccine.Vaccine. 2024 Sep 17;42(22):126165. doi: 10.1016/j.vaccine.2024.126165. Epub 2024 Aug 27. Vaccine. 2024. PMID: 39197299 Review.
Cited by
-
Potent neutralization of SARS-CoV-2 variants by RBD nanoparticle and prefusion-stabilized spike immunogens.NPJ Vaccines. 2024 Oct 8;9(1):184. doi: 10.1038/s41541-024-00982-1. NPJ Vaccines. 2024. PMID: 39379400 Free PMC article.
-
Virological Traits of the SARS-CoV-2 BA.2.87.1 Lineage.Vaccines (Basel). 2024 May 1;12(5):487. doi: 10.3390/vaccines12050487. Vaccines (Basel). 2024. PMID: 38793739 Free PMC article.
-
Passive infusion of an S2-Stem broadly neutralizing antibody protects against SARS-CoV-2 infection and lower airway inflammation in rhesus macaques.bioRxiv [Preprint]. 2024 Jul 30:2024.07.30.605768. doi: 10.1101/2024.07.30.605768. bioRxiv. 2024. PMID: 39109178 Free PMC article. Preprint.
-
COVID-19 mRNA booster vaccination induces robust antibody responses but few adverse events among SARS-CoV-2 naïve nursing home residents.Sci Rep. 2024 Oct 7;14(1):23295. doi: 10.1038/s41598-024-73004-8. Sci Rep. 2024. PMID: 39375365 Free PMC article.
References
-
- US Food and Drug Administration . Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Available at: https://labeling.pfizer.com/ShowLabeling.aspx?id=14471&format=pdf. Accessed 4 December 2022.
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

