A 12-month follow-up of the immune response to SARS-CoV-2 primary vaccination: evidence from a real-world study
- PMID: 38077369
- PMCID: PMC10698351
- DOI: 10.3389/fimmu.2023.1272119
A 12-month follow-up of the immune response to SARS-CoV-2 primary vaccination: evidence from a real-world study
Abstract
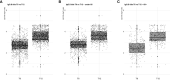
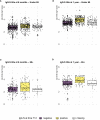
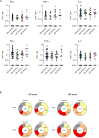
A real-world population-based longitudinal study, aimed at determining the magnitude and duration of immunity induced by different types of vaccines against COVID-19, started in 2021 by enrolling a cohort of 2,497 individuals at time of their first vaccination. The study cohort included both healthy adults aged ≤65 years and elderly subjects aged >65 years with two or more co-morbidities. Here, patterns of anti-SARS-CoV-2 humoral and cell-mediated specific immune response, assessed on 1,182 remaining subjects, at 6 (T6) and 12 months (T12) after the first vaccine dose, are described. At T12 median anti-Spike IgG antibody levels were increased compared to T6. The determinants of increased anti-Spike IgG were the receipt of a third vaccine dose between T6 and T12 and being positive for anti-Nucleocapside IgG at T12, a marker of recent infection, while age had no significant effect. The capacity of T12 sera to neutralize in vitro the ancestral B strain and the Omicron BA.5 variant was assessed in a subgroup of vaccinated subjects. A correlation between anti-S IgG levels and sera neutralizing capacity was identified and higher neutralizing capacity was evident in healthy adults compared to frail elderly subjects and in those who were positive for anti-Nucleocapside IgG at T12. Remarkably, one third of T12 sera from anti-Nucleocapside IgG negative older individuals were unable to neutralize the BA.5 variant strain. Finally, the evaluation of T-cell mediated immunity showed that most analysed subjects, independently from age and comorbidity, displayed Spike-specific responses with a high degree of polyfunctionality, especially in the CD8 compartment. In conclusion, vaccinated subjects had high levels of circulating antibodies against SARS-CoV-2 Spike protein 12 months after the primary vaccination, which increased as compared to T6. The enhancing effect could be attributable to the administration of a third vaccine dose but also to the occurrence of breakthrough infection. Older individuals, especially those who were anti-Nucleocapside IgG negative, displayed an impaired capacity to neutralize the BA.5 variant strain. Spike specific T-cell responses, able to sustain immunity and maintain the ability to fight the infection, were present in most of older and younger subjects assayed at T12.
Keywords: SARS-CoV-2; T-cell; immune response; serology; vaccines.
Copyright © 2023 Fedele, Schiavoni, Trentini, Leone, Olivetta, Fallucca, Fiore, Di Martino, Abrignani, Baldo, Baldovin, Bandera, Clerici, De Paschale, Diaco, Domnich, Fortunato, Giberti, Gori, Grifantini, Lazzarotto, Lodi, Mastroianni, Prato, Restivo, Vitale, Brusaferro, Merler, Palamara, Stefanelli and the Study Group for the Immunological Monitoring post Covid19 vaccination.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Robust Vaccine-Induced as Well as Hybrid B- and T-Cell Immunity across SARS-CoV-2 Vaccine Platforms in People with HIV.Microbiol Spectr. 2023 Jun 15;11(3):e0115523. doi: 10.1128/spectrum.01155-23. Epub 2023 May 11. Microbiol Spectr. 2023. PMID: 37166335 Free PMC article.
-
Long-term quantitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV (PWH).BMC Infect Dis. 2022 Sep 21;22(1):744. doi: 10.1186/s12879-022-07737-0. BMC Infect Dis. 2022. PMID: 36131232 Free PMC article.
-
BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers.Lancet Respir Med. 2021 Sep;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4. Epub 2021 Jul 2. Lancet Respir Med. 2021. PMID: 34224675 Free PMC article.
Cited by
-
Appropriate Sampling and Longer Follow-Up Are Required to Rigorously Evaluate Longevity of Humoral Memory After Vaccination.Immunohorizons. 2024 Jun 1;8(6):397-403. doi: 10.4049/immunohorizons.2300057. Immunohorizons. 2024. PMID: 38864816 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

