A novel mechanism of PHB2-mediated mitophagy participating in the development of Parkinson's disease
- PMID: 38103250
- PMCID: PMC10960274
- DOI: 10.4103/1673-5374.389356
A novel mechanism of PHB2-mediated mitophagy participating in the development of Parkinson's disease
Abstract
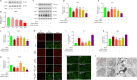
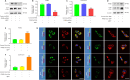
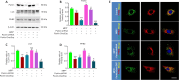
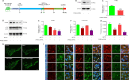
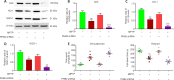
JOURNAL/nrgr/04.03/01300535-202408000-00037/figure1/v/2023-12-16T180322Z/r/image-tiff Endoplasmic reticulum stress and mitochondrial dysfunction play important roles in Parkinson's disease, but the regulatory mechanism remains elusive. Prohibitin-2 (PHB2) is a newly discovered autophagy receptor in the mitochondrial inner membrane, and its role in Parkinson's disease remains unclear. Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) is a factor that regulates cell fate during endoplasmic reticulum stress. Parkin is regulated by PERK and is a _target of the unfolded protein response. It is unclear whether PERK regulates PHB2-mediated mitophagy through Parkin. In this study, we established a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of Parkinson's disease. We used adeno-associated virus to knockdown PHB2 expression. Our results showed that loss of dopaminergic neurons and motor deficits were aggravated in the MPTP-induced mouse model of Parkinson's disease. Overexpression of PHB2 inhibited these abnormalities. We also established a 1-methyl-4-phenylpyridine (MPP+)-induced SH-SY5Y cell model of Parkinson's disease. We found that overexpression of Parkin increased co-localization of PHB2 and microtubule-associated protein 1 light chain 3, and promoted mitophagy. In addition, MPP+ regulated Parkin involvement in PHB2-mediated mitophagy through phosphorylation of PERK. These findings suggest that PHB2 participates in the development of Parkinson's disease by interacting with endoplasmic reticulum stress and Parkin.
Copyright © 2024 Copyright: © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures
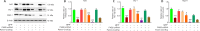
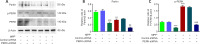
Similar articles
-
Nonreceptor Tyrosine Kinase c-Abl-Mediated PHB2 Phosphorylation Aggravates Mitophagy Disorder in Parkinson's Disease Model.Oxid Med Cell Longev. 2022 Nov 9;2022:9233749. doi: 10.1155/2022/9233749. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36406767 Free PMC article.
-
Teaghrelin protected dopaminergic neurons in MPTP-induced Parkinson's disease animal model by promoting PINK1/Parkin-mediated mitophagy and AMPK/SIRT1/PGC1-α-mediated mitochondrial biogenesis.Environ Toxicol. 2024 Jul;39(7):4022-4034. doi: 10.1002/tox.24275. Epub 2024 Apr 15. Environ Toxicol. 2024. PMID: 38622810
-
Rutin Attenuates Oxidative Stress Via PHB2-Mediated Mitophagy in MPP+-Induced SH-SY5Y Cells.Neurotox Res. 2023 Jun;41(3):242-255. doi: 10.1007/s12640-023-00636-5. Epub 2023 Feb 4. Neurotox Res. 2023. PMID: 36738374
-
Organelle-specific autophagy in inflammatory diseases: a potential therapeutic _target underlying the quality control of multiple organelles.Autophagy. 2021 Feb;17(2):385-401. doi: 10.1080/15548627.2020.1725377. Epub 2020 Feb 12. Autophagy. 2021. PMID: 32048886 Free PMC article. Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Recent progress in the applications of presynaptic dopaminergic positron emission tomography imaging in parkinsonism.Neural Regen Res. 2025 Jan 1;20(1):93-106. doi: 10.4103/1673-5374.391180. Epub 2023 Dec 21. Neural Regen Res. 2025. PMID: 38767479 Free PMC article.
References
-
- Almeida LM, Pinho BR, Duchen MR, Oliveira JMA. The PERKs of mitochondria protection during stress: insights for PERK modulation in neurodegenerative and metabolic diseases. Biol Rev Camb Philos Soc. 2022;97:1737–1748. - PubMed
-
- Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trümbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF. Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ. 2011;18:769–782. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

